-
Previous paper
Zooplankton of Moreton Bay
Sarah Pausina1,2 Jack Greenwood3, Kylie Pitt4, David Rissik5,6, Wayne Rochester2, Jennifer Skerratt7, Julian Uribe-Palomino2 and Anthony J. Richardson2,8 -
This paper
Coral and micro-benthic assemblages from reef habitats in Moreton Bay
John M. Pandolfi1, Matt Lybolt2, Brigitte Sommer3, Roshni Narayan4, Paola Rachello-Dolmen5 -
Next paper
Fishes of Moreton Bay: Ecology, human impacts, and conservation
Andrew D. Olds1, Ben L. Gilby1, Rod M. Connolly2, Ian R. Tibbetts3, Christopher J. Henderson1, Tim Stevens2, Sarah K. Thackwray1, and Thomas A. Schlacher1
Coral and micro-benthic assemblages from reef habitats in Moreton Bay
Authors
John M. Pandolfi1, Matt Lybolt2, Brigitte Sommer3, Roshni Narayan4, Paola Rachello-Dolmen5Author affiliations
- ARC Centre of Excellence for Coral Reef Studies and School of Biological Sciences, The University of Queensland, St Lucia Queensland, 4072, Australia;
- CARDNO, Pacific Guardian Center, 737 Bishop Street, Mauka Tower, Suite 3050, Honolulu, HI 96813, USA;
- School of Life and Environmental Sciences, The University of Sydney, Sydney NSW, 2006, Australia;
- Ocean Frontier Institute, Halifax, Nova Scotia, Canada;
- ELSEVIER, The Hague Area, Netherlands.
Corresponding author
j.pandolfi@uq.edu.auORCID
John Pandolfi: https://orcid.org/0000-0003-3047-6694
Matt Lybolt: https://orcid.org/0000-0002-7175-7014
Brigitte Sommer: https://orcid.org/0000-0003-0617-7790
Paola Rachello-Dolmen: https://orcid.org/0000-0002-2056-9101

Book
Coral and micro-benthic assemblages from reef habitats in Moreton Bay
Chapter
Research Paper Title
Coral and micro-benthic assemblages from reef habitats in Moreton Bay
Cite this paper as:
Pandolfi J, Lybolt M, Sommer B, Narayan R, Rachello-Dolmen P. 2019. Coral and micro-benthic assemblages from reef habitats in Moreton Bay. In Tibbetts, I.R., Rothlisberg, P.C., Neil, D.T., Homburg, T.A., Brewer, D.T., & Arthington, A.H. (Editors). Moreton Bay Quandamooka & Catchment: Past, present, and future. The Moreton Bay Foundation. Brisbane, Australia. Available from: https://moretonbayfoundation.org/
DOI
10.6084/m9.figshare.8074328
ISBN
978-0-6486690-0-5
Abstract
The subtropical coral reefs of Moreton Bay support a rich diversity of corals and micro-benthic organisms. These high-latitude reef communities exist in marginal environments that include relatively cooler, more light-limited, and more variable environmental conditions than those in the tropics. Holocene reef coral communities formed episodically over the Bay’s 7000-year history, with a high degree of persistence in community structure and reef accretion rate until European colonisation of the Queensland coastline. However, during the most recent phase of the Bay’s reef development, reductions in water quality have transformed the Bay’s coral assemblages from predominantly large, fast-growing and branching acroporid corals to predominantly slower growing and smaller massive corals. The modern composition and diversity of benthic foraminiferal and micro-molluscan communities is driven mainly by substrate and water-quality parameters and shows a striking gradient from the variable and stressed water conditions of the western Bay to the more open-marine higher water quality habitats of the eastern Bay, including Myora Reef. Episodic changes also occurred in the Holocene benthic microfaunal composition, confirming the fluctuating nature of the Bay’s marine environments. Recent increases in foraminifera diversity and symbiont-bearing taxa signals a subtle improvement in water quality from the 1970s to 2008; however, for micro-gastropods, comparisons between fossil and modern death assemblages illustrate a decline in the condition of modern Bay habitats. The Holocene variation in the taxonomic composition and diversity of coral and micro-benthic assemblages of Moreton Bay reveals a history of recovery and rapid reef growth. Rapid recovery may still be possible if the causes of anthropogenic degradation are reversed and for this the highest priority is to reduce sediment and nutrient delivery into the Bay’s marine habitats.
Keywords: marginal reefs, high-latitude reefs, coral reefs, Australia, palaeoecology, land-use changes
Introduction
As tropical coral reefs worldwide are threatened by over-exploitation and climate change (1–5), the ecological importance of high-latitude reefs and reefal communities has become more recognized, both as potential refuge areas for tropical species (6–8) and for their inherent ecological values. While tropical coral reefs tend to occur in warm, clear, shallow, oligotrophic, fully saline and aragonite supersaturated seas, high-latitude coral reef assemblages exist at the margins of species distributions and environmental tolerances, particularly for temperature, light availability and aragonite saturation (9–11). High-latitude coral communities are primarily non-reef building (though exceptions occur), and are distinguished from framework building coral reefs in a geological sense by their inability to accrete calcium carbonate reefs (12). Instead, they commonly grow as low-relief veneers of living coral on non-reefal substrates that follow the existing seafloor morphology (13). High-latitude reef communities are also characterized by a unique biogeographic overlap with other benthic organisms, many with temperate distributions (Sommer unpubl.) (14–16). High-latitude reefs therefore fit the definition of marginal reefs in several ways, as they occur where biodiversity patterns, environmental conditions and ecosystem function differ substantially from those associated with ‘classical’ tropical coral reefs (9, 17, 18). High-latitude reefs generally occur above 23.5° latitude (9) in a range of locations around the globe (see Fig. 1 in 14). In the southern hemisphere the highest latitude true coral reefs (i.e. framework building, accreting) are located at 31°33’S at Lord Howe Island, Australia (19, 20) and at 33°48’N at Iki Island, Japan, (21) in the northern hemisphere (22).
Located ~400 km south of the southernmost cay (Lady Elliot Island) on the Great Barrier Reef and adjacent to a major city, the reefs of Moreton Bay provide a rare instance of subtropical, marginal coral reefs in an urbanised environment (23). Abundant coral communities and reefs have characterised the history of Moreton Bay, South East Queensland. These shallow-water coral assemblages have experienced episodic reef growth throughout the Holocene related to periods of sea-level and natural climate change. Living corals now grow as a veneer on these Holocene carbonate deposits (23). Indeed, the Moreton Bay Region has the highest coral diversity along the eastern Australian subtropical-to-temperate transition zone, with a marked reduction in species richness further south (15, 16, 24, 25). Some high-latitude reefs, such as Flinders Reef at 26°58’S in Outer Moreton Bay, harbour rich coral faunas (e.g. 125 scleractinian species from 35 genera (23), and are important stepping stones for tropical coral species in this biogeographic transition zone.
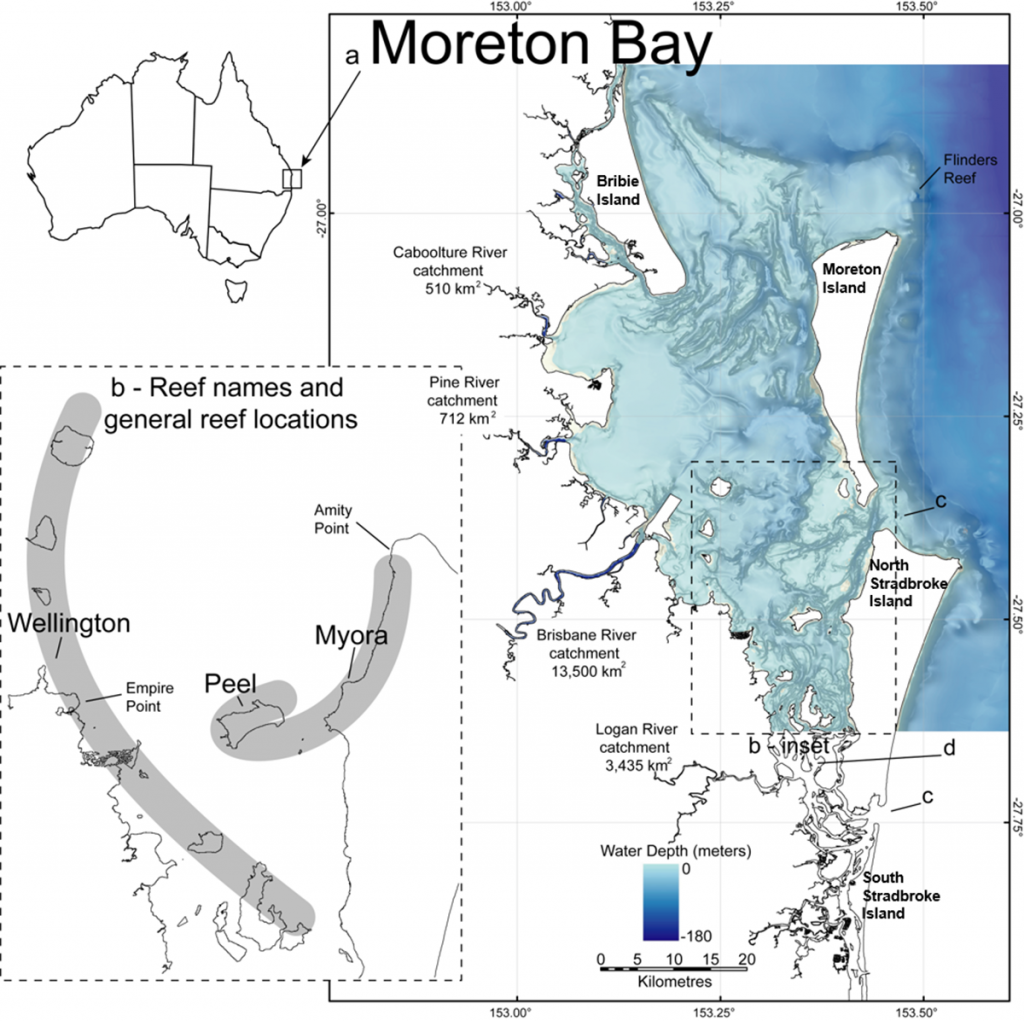
In this paper, we focus on three components of subtropical reefal biodiversity — corals, large benthic foraminifera and benthic micro-gastropods — because they are easily fossilisable and thus provide important clues as to the history of Moreton Bay. Though we provide some information about the Bay in general, we focus on ecological patterns that have been compared between modern and ancient settings from western, central and eastern sites within the Bay: Wellington Point, Peel Island and Myora Reef. We follow the Wallace et al (23) geographic separation of Moreton Bay into an inner region composed of the body of water partly enclosed by North and South Stradbroke, Moreton, and Bribie islands, and an outer region composed of the rocky reefs immediately outside these large islands, including Flinders Reef near Moreton Island and Flat Rock, Shark Gutter and Shag Rock off the north-east corner of North Stradbroke Island. Further information on the geological history of reef habitats in Moreton Bay is found in Lybolt & Pandolfi 2019, this volume (26).
Contemporary diversity of corals and benthic microfauna
Corals
Coral species richness and community composition in the Bay is variable in space and time. The spatial pattern of richness generally follows the dominant water-quality gradient from poor water-quality reefs with lower species richness near the mainland to better water-quality reefs with greater richness towards the oceanic inlets in the east (27–29). The modern reefs in the inner Bay exhibit reduced cementation, coral cover (2–30%) and richness (S=64 species from 26 genera), compared to Flinders Reef from the outer Bay, which contains 28–40% cover and higher richness (S=125 species from 35 genera) (Fig. 1) (23). In inner Moreton Bay, based on current taxonomy, Merulinidae is the dominant coral family, followed by Lobophylliidae, Acroporidae and Dendrophylliidae, while in outer Moreton Bay Acroporidae are dominant based on the number of species and genera present (23).
The modern reefs of outer Moreton Bay (Fig. 1) host about half as many species as Lord Howe Island, a pattern generally following water-quality gradients from exceptional at Lord Howe to poor in Moreton Bay (29), though other factors such as substrate quality and water circulation also play a part in this distribution (15). Although the inner/outer Bay distribution of species suggests a nested pattern of richness, 18 inner Moreton Bay species are not found in the outer Moreton Bay reefs. Therefore, winnowing out the less hardy species is not the only major process driving differences in coral assemblage composition — some degree of local adaptation coupled with variance in competition or other aspects of biotic interactions or niche constraints are also likely to be involved.
Recent studies show that, although the coral species on high-latitude reefs of eastern Australia generally also occur on the Great Barrier Reef, high-latitude coral assemblages tend to have narrower taxonomic, functional and phylogenetic breadth than coral assemblages on the Great Barrier Reef (10, 24, 30). High-latitude reefs are characterised by widely distributed, generalist, stress-tolerant and opportunistic coral species with massive and horizontally spreading morphologies and by diminishing influence of tropical taxa at higher latitudes (24). Flinders Reef in outer Moreton Bay is the exception, with broad areas showing high abundance of the tall, branching species Acropora intermedia (24, 30), which tends to be common on tropical coral reefs (31, 32) and absent or rare at other reefs in the eastern Australian subtropical-to-temperate transition (24, 30). Using a biogeographic classification of corals into ‘tropical’, ‘subtropical’ and ‘cosmopolitan’ species, Sommer et al (24) showed that Flinders Reef and Flat Rock in outer Moreton Bay have the greatest abundance of tropical corals on rocky reefs south of the Great Barrier Reef, with cosmopolitan and subtropical species dominant on rocky reefs in New South Wales (24).
Environmental tolerance is important for corals in these marginal environments, and species with unsuitable traits cannot persist in the relatively cooler, more light-limited and more variable environmental conditions of the subtropics (10, 24, 30). Coral species unable to tolerate these marginal conditions are excluded from high-latitude reefs. The important role of abiotic processes in structuring high-latitude coral communities is further supported by strong relationships between coral biodiversity patterns and environmental gradients in the region (10). Climate envelope modelling shows that the relative importance of environmental drivers varies among species (10). Light availability best explains gradients in species richness and the distribution of tropical corals, whereas cold stress and temperature variability best explained gradients in Shannon diversity, functional diversity and the distribution of subtropical coral species (10). Nevertheless, the dominant influence of abiotic filtering at high-latitudes does not diminish the importance of biotic interactions (e.g. competition) on high-latitude reefs. Indeed, patterns in phylogenetic diversity suggest that species interactions (e.g. competition for space or light) are also important drivers of biodiversity patterns at local scales, such as at Flinders Reef (30), where corals with a ‘competitive’ life-history strategy (i.e. large, branching and plating species that grow quickly, occur at shallow depths and reproduce by broadcast spawning; 33) were particularly abundant (24, 30).
Benthic microfauna
Benthic foraminifera
Benthic foraminifera are abundant and widely distributed in marine sediments across a broad range of marine environments (intertidal sand and mud flats, seagrass meadows and coral reefs) in Moreton Bay (34–36). The foraminiferal species abundance, diversity and community composition vary spatially along a (west-to-east) gradient in water and substrate quality, from nearshore, riverine-influenced to near-oceanic and well-flushed conditions (34). This environmental gradient drives differences in foraminiferal species composition with a total of 69 benthic foraminiferal species representing relatively low Shannon–Wiener species diversity (H´=1.4 to 2.2) and Margalef’s richness index (d=0.9 to 5.9) (34). Overall, the foraminiferal species composition, low diversity assemblages and the resulting FORAM Index values, a metric for determining water quality (based on the relative proportion of three functional groups of foraminifers: opportunistic, heterotrophic and symbiont-bearing large benthic foraminifers) (37), are consistent with the prevalence of contemporary, turbid, eutrophic–mesotrophic, marginal marine conditions in Moreton Bay (34, 38).
Narayan & Pandolfi. 2010 (34) found a significant positive relationship between community composition and sediment types from the Bay-wide environments. Foraminiferal communities that occurred in the muddy, (coarse-grained) quartz sand sediments of the westernmost, nearshore, riverine-influenced environments were compositionally different from the communities found in the calcareous sands-muds and coralgal (reef flat) rubble of the western-central Bay and also from the clean, quartz and calcareous sand sediments from the (reef- and seagrass-dominated) tidal delta flats of the eastern Bay (34). The species diversity was found to be higher in western Waterloo Bay (e.g. Wellington Point, Green and St. Helena islands), central Bay (Peel Island) and the eastern Bay (north of Peel Island and south-western Moreton Island) than in the Brisbane River delta or Deception Bay environments (34). The latter environments are highly influenced by estuarine conditions and impacted by sediment run-off (34, 39, 40). However, in contrast to species composition, sediment types did not significantly influence species diversity, even though species richness was greatly reduced in the nearshore-riverine sediments compared with the other sediment types (muddy sands, sandy muds, clean tidal sands, biogenic sands) encountered in the Bay (34).
Cosmopolitan, opportunistic foraminiferal taxa (i.e. Ammonia spp. and Elphidium spp.), which can tolerate a wide range of water quality, salinities and (low) oxygen conditions (37, 41, 42), overwhelmingly dominated (with a frequency of occurrence of 92% and 80%, respectively) in the marine sediment samples of Moreton Bay (34). The relatively stress tolerant Ammonia sp. cf. A. aoteana (formerly A. beccarrii in (34)) and other agglutinated taxa dominated the biocenosis (assemblage) of the westernmost nearshore, riverine-influenced environments (34). Generally, this assemblage corresponds with low mean species diversity (d=1.4±0.2) and extremely low mean FORAM Index values (≈1) reflecting poor water and sediment quality (34). Moving westward, the large, shallow-water, opportunistic-mixotrophic species Elphidium craticulatum (and E. discoidales multiloculum) dominates the biocenosis of the calcareous muds and sand sediments of the seagrass meadows and marginal reefs, which occur from the western Waterloo Bay (Wellington Point, Green and St. Helena islands) to the central Bay (Peel Island) region (34). The abundance of mixed opportunistic-heterotrophic (miliolinids) assemblage corresponds with a slightly higher mean species diversity (d=2.2±0.3) and low mean FORAM Index values (≈2), which again indicate variable and stressed water quality conditions generally not conducive to reef growth, despite the presence of patchy coral colonies (23, 28, 38, 40, 43). The cosmopolitan symbiont-bearing large benthic foraminifera (LBFs, i.e. Peneroplis spp., Alveolinella quoyi), which are indirect indicators of clear water-quality conditions, increased in average percent relative abundance (3.5±3.7 to 62.2±12.4) and dominated the biocenosis of the tidal sand flats of the eastern Bay, where abundant seagrass meadows supported LBF epiphytes (34). In the eastern Bay, the increased abundance of LBFs corresponds with a mean species diversity of d = 2.1±0.2 and a significantly improved FORAM Index value (7.6). This improved water quality corresponds with the occurrence of low diversity, water-quality sensitive, fast-growing Acropora coral communities found to occur in the eastern Bay locality of Myora Reef (Fig. 1).
Benthic micro-gastropods
Information on the nature and distribution of marine micro-molluscs and the linkages between different habitats are rare (44, 45), and this is particularly true for micro-gastropods in subtropical estuaries in Australia (46). In Moreton Bay, recent analysis of micro-gastropod relative abundance data showed that gastropod assemblage distribution is driven mainly by substrate and water-quality parameters (turbidity, total nitrogen, temperature and dissolved oxygen) (47). Rachello-Dolmen (47) identified 219 species belonging to 115 genera and 46 families from modern death assemblages (grab samples) from Moreton Bay. The variation in the distribution of taxonomic composition of micro-gastropod assemblages in the Bay is in part explained by substrate, total nitrogen, temperature, turbidity and depth. Environmental conditions were associated with species traits: (i) species with discoidal shape and nodulose sculpture exhibited the most pronounced preference for western sites, near the Brisbane River, linked with turbidity and high nitrogen concentrations; (ii) ectoparasite species on echinoderms or actinarians, anemones and corals were associated with western central Bay sites associated with high phosphorous and chlorophyll a; (iii) tropical, subtropical and temperate species of small to medium size, fusiform shape, carnivorous, and found in the lower intertidal zone were associated with eastern central Bay sites linked with good light penetration, high oxygen concentrations and normal marine salinity; and (iv) globose species of medium size, herbivore- grazers found in the upper intertidal zone exhibited a preference for eastern sites, away from the Brisbane River mouth, with relatively constant temperature and pH. Water temperature plays a significant role in driving changes in rare species. The rare species were found mostly at Peel Island (17 species) and Myora Reef (7 species) where recruitment is apparently sustained by the influx of the East Australian Current through the South Passage between Moreton and North Stradbroke island (48, 49).
Holocene marine benthic faunas
Holocene coral communities
Coral richness, for both genera and species, is at its highest today (S=64 (23)), and was lower in the early 1970s (S=24 (50)) and in the early 1950s (S=24 from 12 genera (51)). Earlier surveys listed a number of species and described coral communities that seem similar to today including Myora’s unique Acropora-dominated community (52–54). Changes in the coral assemblage over longer temporal scales are already known for some genera, although the timing of the changes is poorly understood. The fossil reefs of the Bay were first shown to host 36 species from 20 genera (51), and the single Pleistocene reef discovered in the Bay included 39 species from 25 genera (55). In the rough time frame of modern, Holocene and Pleistocene only 10 genera are common to all three. For example, Montipora is known from the Pleistocene and Holocene fossil deposits but is absent from the living coral assemblage in Moreton Bay, although it is present on artificial structures near oceanic inlets (23, 51, 55). Nonetheless, the modern assemblage of 26 genera is greater than both Holocene (20 genera) and the single Pleistocene (25 genera) fossil reef assemblages.

Coral reef development in the Bay was episodic during the past 7,000 years of the Holocene, when both the taxonomic composition of the coral assemblages and reef accretion rates of Moreton Bay were striking for their consistency over time (26, 56). Within each reef site of the Bay, there is no change in the taxonomic composition of coral assemblages over time and no change in reef accretion rate over time (Fig. 2, (56)).
Holocene benthic microfauna
Benthic foraminifera
Spatio-temporal distribution patterns and species composition of the benthic foraminifer microfauna derived from Holocene sediment deposits were assessed from three sites (Wellington Point, Peel Island and Myora Island) along a west to east gradient of water quality in Moreton Bay (34, 57). The results showed that low-diversity and low-density assemblages of benthic foraminifers dominated throughout the Holocene, between 0.4 to 7.4 cal ka yBP (57). However, episodic changes in the microfaunal and sediment composition confirm the highly fluctuating nature of the Bay’s marine environments, since the onset of reef initiation (as represented by pre-Holocene terrestrial basal sedimentary layers) (38, 57). The episodic disruptions in reef sediment deposition (38) are supported by shifts in the foraminiferal assemblages, which are associated with sedimentological (biofacies) changes over time (57), despite Holocene sea level being ~ 1.5 to 2 m higher than today. For example, reef sediment cores from Wellington Point clearly document a transition from older (3.9–4.9 cal ka yBP) reefal carbonate mud and sands, rich in foraminifer shells, to younger (3.6–3.7 cal ka yBP) clean medium-course shell hash/sands, with minor occurrences of foraminifer shells, indicating a shoaling event from a deep, subtidal (depositional) reef slope setting to a (non-depositional) intertidal environment over time (57).
Holocene foraminiferal species composition consistently displayed a relatively high abundance (~60%) of foraminiferal assemblages dominated by miliolid, small-heterotrophic taxa (e.g. Quinqueloculina spp., Spiroloculina spp. and Triloculina spp.) (57). This is indicative of normal marine conditions, likely as a result of higher sea levels than today (57). The stress-tolerant rotalid, opportunistic taxa (e.g. Ammonia sp. cf. A. aoteana, Elphidium craticulatum and E. hispidulum) followed in abundance (~20–30%) (57). For example, the large, mixotrophic-opportunistic E. craticulatum was found to dominate in the shallow-water (2 m) intertidal reef flats during the Holocene and today (34, 57). In contrast, low percentages (~ <10%) of symbiont-bearing large benthic foraminifers (LBFs, predominantly epiphytic Peneroplis spp.) were recorded, with their highest frequency in the eastern south-west Peel Island and Myora Island reef sites (57). The symbiont-bearing LBF assemblage, indicative of good water- quality conditions optimal for coral reef growth, and their predominance in the eastern Bay reefs and tidal flats is comparable to their distribution today (57). Over time, the FORAM Index value remained below 4 in the western Wellington Point Reef, indicating the prevalence of variable but mostly marginal marine conditions throughout the Holocene (57). However, the FORAM Index at times exceeded 4 in the south-west Peel Island and Myora Island reefs, suggesting episodic improvements in water quality (57).
Benthic changes since European colonisation
Coral community changes
The coral reefs of Moreton Bay exhibited robust growth in the mid-Holocene that gradually declined in the absence of major anthropogenic disturbance, but recently exhibited significant modern degradation (4, 28, 38). Previous studies of the marginal reefs of Moreton Bay have proposed that changes in temperature, sea level, El Niño–Southern Oscillation intensity, and sedimentation led to natural reef declines sometime between 3000 and 8000 years ago (28), prior to major anthropogenic disturbance. Further decline, due to over-exploitation and water-quality degradation, has been indicated since European settlement began in 1824 (4, 40).
Anthropogenic stressors in the Bay and its catchment occurred in three periods closely tied to land-use practices. The early and mid-Holocene probably had negligible cumulative anthropogenic impacts aside from small weirs built as fish traps (58). During the second period, the late Holocene, the primary anthropogenic impact was selective burning to promote grasslands for hunting (40, 59–61). This practice, termed ‘firestick farming’, was only possible after ~5000 ybp because the enhanced mid-Holocene precipitation regime prevented most fires (60, 62). Increased erosion and sedimentation associated with natural fires and burning by Aboriginal people would have impacted the Bay, but the magnitude of this impact probably did not increase through the late Holocene because fire frequency did not increase during this time (62). During the third period, rapidly accumulating anthropogenic impacts followed European settlement from the early 19th century, ~ 1850 CE to present, particularly land-use changes that enhanced erosion by 2–10 times (63, 64), direct impacts of coastal construction, over-harvesting, and increased nutrient loading from husbandry, agriculture and sewage (4, 28, 45, 65). The naturalist and photographer Saville-Kent noted the coral mining in Moreton Bay and wrote that ‘By these means, beyond doubt, the original abundant growth of coral in this special area has been materially diminished’ (52 p96). Direct harvest of Moreton Bay corals for use as building materials persisted until 1997 (66).
The modern coral assemblages of Moreton Bay are strikingly different from the fossil assemblages (23, 28, 38). The modern assemblage is dominated by massive, encrusting and foliose genera such as Favia, Goniastrea, Goniopora and Turbinaria, whereas branching Acropora is uncommon. Dominance in the fossil assemblage is reversed, and branching Acropora is a dominant component of the Holocene assemblage (23, 38). This distinction was noted very early in the study of the Bay (52, 53) and first quantified by Wells (51). Subsequent research in Moreton Bay confirmed these observations and included constraints on the potential timing of the change from branching to massive-coral-dominated assemblages. Flood (67) attributed the shift to a ≈1 m sea-level fall estimated at 4000–3000 years ago. Johnson and Neil (28) attributed the shift to synergistic impacts starting 5500 years ago and progressively worsening to modern times. However, Lybolt et al. (38) and Lybolt (56) demonstrated that the shift occurred in modern times. There was no evidence of the assemblage shift while reefs were growing from 7400–165 years ago, but sometime between 165 and 52 years ago (1852 and 1956 CE) most reefs in the Bay ceased to be Acropora-dominated.
The timing of this unprecedented change between fossil and modern assemblages strongly suggests a modern anthropogenic cause. European settlement of the area began in 1824, and within decades radical landscape changes and habitat destruction caused more than a threefold increase in sedimentation to nearshore waters (39, 63, 68). Furthermore, the modern Acropora-dominated assemblage at Myora, a living remnant of the Bay’s formerly typical coral assemblage, demonstrates that water quality near the oceanic inlets remains suitable for this assemblage. This shift in community dominance is unlikely to indicate senescence (69) because it is recorded across the depth range of corals in the Bay (29), rather than confined to those shallowest portions of the reef most affected by reduced accommodation space. The trajectory of decline in Moreton Bay, however, is not monotonic. Wells (51) found three genera living but absent in the fossil reefs, and nine genera in the fossil reefs but absent from the living assemblage. In 1955, the fossil coral assemblages were richer than the living coral assemblages. The situation today is reversed. Wallace et al. (23) found seven (colonial) coral genera in the living assemblages but absent from the fossil assemblage within Moreton Bay, while there are only four (colonial) coral genera found in the fossil reefs but absent from the living assemblage. While this apparent increase in Moreton Bay coral diversity since 1955 is not significant (56), a potential reversal of a trajectory of anthropogenic degradation would be encouraging, and seldom seen in studies of historical range of variation (4) (though see Hawaii example in (70)). Alternatively, more recent higher diversity might also stem from temperature increases allowing successful recruitment of larvae arriving from the north.
Benthic microfauna changes
Benthic foraminifera
The calcium carbonate tests of benthic foraminifers are well preserved and abundant in shallow to deep-water marine sedimentary deposits worldwide (71, 72). They have been used as important ecosystem bio-indicators for reconstructing present and past environmental changes and in understanding the baseline conditions prior to significant human-induced impacts in the coastal marine environments of eastern Australia (57, 73). A comparison of foraminiferal species composition data between samples collected by the Geological Survey of Queensland in the 1970s (36) and samples collected in 2008 revealed significant differences in species composition (74). The finding of an increase in diversity in the Brisbane River delta and the Waterloo Bay areas and the presence of epiphytic, symbiont-bearing LBF taxa (Peneroplis spp.) in the Waterloo Bay area signals a subtle improvement in water quality in the latter period, a surprising result considering that the catchment now hosts more than two million people (74).
Benthic micro-gastropods
Comparison of micro-gastropod community data derived from Holocene cores with data collected from modern reefs at the same location has great potential to inform assessments of long-term reef ecological trajectories (75). In Moreton Bay, the taxonomic composition of fossil and modern micro-gastropod assemblages varies significantly (47). At Wellington Point, fossil assemblages are composed predominantly of Rissooidea (biofilm grazer) and modern assemblages by Columbellidae (herbivore or carnivore); both families can live on algae, coral rubble, under stones, soft substrate or seagrass (76). At Peel Island, fossil assemblages are composed predominantly of Rissooidea and modern death assemblages of Scaliolidae (biofilm grazer) and Cerithiidae (detritivore), both families living in soft substrate. Certihiidae also can be found on hard substrates. At Myora Reef, fossil assemblages are composed predominantly of Cerithiidae, Calopiidae (biofilm grazer), Triphoridae and Cerithiopsidae (both ectoparasites of sponges). All these families live on soft or hard substrata and seagrass. Modern death assemblages in Myora are composed predominantly of Trochidae (carnivore or grazer or herbivore) and Columbellidae (carnivore or herbivore), both families can live on algae or hard or soft substrate and seagrass. Data on substrate and feeding type are abundant for gastropod families (76), but environmental and biotic parameters associated with micro-gastropods are unknown.
Differences between fossil and modern death assemblages in the Bay are probably due to the anthropogenic stressors that occurred in the three periods closely tied to land-use practices noted above. During European colonization, fisheries and dredging activities were developed, coinciding with marked ecosystem changes. Relative changes in rare vs intermediate abundance of families can be an indication of human-disturbed environments (77), and in Moreton Bay, micro-gastropods show a decreasing number of rare families coupled with an increasing number of families with intermediate abundance in modern samples compared with Holocene occurrences. The larger number of rare species encountered in the fossil assemblages resulted in slightly higher diversity in fossil assemblages than in modern death assemblages. Patterns of changing diversity and family abundance distribution have the potential to serve as environmental indicators (78), and in Moreton Bay illustrate a decline in the condition of modern reefs (47).
Threats and management considerations
The condition of reefs is directly related to the duration and intensity of human impact on reef systems and, as a consequence, reefs worldwide are threatened by the cumulative effects of overfishing and pollution (4). Moreton Bay is no exception to the effect of these threats. Studies of processes structuring species assemblages and temporal and spatial variation in the fauna are necessary in Moreton Bay as they will have implications for attempts to manage diversity and to monitor trends in the health of Moreton Bay reefs (44, 79). Management strategies that help maintain large populations are likely to best facilitate the continuity of high-latitude reefs and their refuge potential (14). For example, the larger populations of tropical species in South East Queensland than in New South Wales means that tropical corals in South East Queensland are likely to be less dependent on Great Barrier Reef source populations for their replenishment than their New South Wales counterparts. These South East Queensland populations may also provide important stepping stones for higher-latitude reefs located further south (22).
Moreover, larger populations tend to have greater standing genetic variation and to be less susceptible to genetic drift, likely also bestowing higher evolutionary potential (80, 81). A phylogenetic signal found in seven tested coral species traits indicates that environmental tolerances of corals are likely to be stable over time, and that corals will only expand their ranges to regions where environmental conditions are similar to conditions experienced in their core ranges (30). The high abundance of tropical coral species in the Moreton Bay Region (24) therefore suggests that this region has high refuge potential for tropical coral species (22) as Great Barrier Reef populations become threatened by increasing temperature (5). Environmental conditions at outer Moreton Bay sites are less light limited and warmer than sites in New South Wales (10) and probably more favourable for establishment of tropical coral species than higher latitude regions (22).
The key benefit of applied palaeoecology is improved natural resource management planning and setting goals that consider the region’s history. Reefs in Moreton Bay grew episodically over 7000 years with no significant change in community composition or accretion rate. However, in the past 200 years the coral species composition of Bay reefs changed substantially, and for the first time in 7000 years the corals of Moreton Bay persist in a degraded state caused by increased sediment and nutrient run-off from anthropogenic land-use changes (38). This means that natural resource managers hoping to reverse this degraded state should target any prescription that reduces sediment and nutrient loads onto the reefs. The historically relevant indicator of success, over the short-term, is any increase in the abundance of Acropora (i.e. increases in the abundance of Acropora indicate the success of natural resource management actions to improve water quality in the catchment). Even a marginal reef habitat such as Moreton Bay has a history of recovery and rapid reef growth, and rapid recovery may yet be possible if the causes of anthropogenic degradation are reversed.
A review of active and passive management initiatives suggests that stringent protection of reefs in no-take marine protected areas is critical to foster ecosystem resilience and refuge potential of high-latitude reefs (14). Moreton Bay reefs are currently protected in marine parks, however a concerning trend in recent years has been a reduction in the level of protection and size of protected areas along the subtropical-to-temperate transition zone; a trend that needs to be urgently reversed to maximise marine park effectiveness (82).
Effective coastal marine management practices that aim to preserve ecosystem services can benefit from the incorporation of relevant marine bio-indicators, which can provide an indirect link between environmental conditions such as water quality and ecosystem (coral reef) health (83, 84). Micro- and meio-benthic organisms, especially benthic foraminifers, have been successfully applied in short- and long-term water quality and coral reef health assessments by several studies including those from Moreton Bay and the Great Barrier Reef (34, 73, 85–87). Foraminifers are considered high-priority bio-indicators for long- and short-term monitoring programs (37, 42, 83){Hallock, 2012 #1435}. While the necessity for taxonomic expertise has been considered the main limiting factor in the application of foraminifers to ecological monitoring practice, developing and successfully establishing foraminiferal metrics such as the FORAM Index (37) has made it possible for researchers and/or marine managers with limited taxonomic expertise to quantify species composition. In addition, foraminifer bio-indicators provide a means for quick, low-cost collection with a low ecological footprint where there are limited technological resources available for monitoring (37, 42). Their continued application for assessing short- and long-term environmental changes in Moreton Bay, along with studies of benthic micro-gastropods, is highly recommended.
References
- Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS. 2010. Rising to the challenge of sustaining coral reef resilience. Trends in Ecology & Evolution. 25(11): 633-642
- Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. 2011. Projecting coral reef futures under global warming and ocean acidification. Science. 333(6041):418-422
- Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J. 2003. Climate change, human impacts, and the resilience of coral reefs. Science. 301(5635):929-933
- Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC. 2003. Global trajectories of the long-term decline of coral reef ecosystems. Science. 301(5635):955-958
- Hughes TP, Kerry JT, Alvarez-Noriega M, Alvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JB, Harrison HB, Heron SF, Hoey AS, Hobbs JPA, Hoogenboom MO, Kennedy EV, Kuo CY, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK. 2017. Global warming and recurrent mass bleaching of corals. Nature. 543(7645):373-377
- Greenstein BJ, Pandolfi JM. 2008. Escaping the heat: range shifts of reef coral taxa in coastal Western Australia. Global Change Biology. 14(3):513-528
- Makino A, Yamano H, Beger M, Klein CJ, Yara Y, Possingham HP. 2014. Spatio-temporal marine conservation planning to support high-latitude coral range expansion under climate change. Diversity and Distributions. 20(8):859-871
- Yamano H, Sugihara K, Nomura K. 2011. Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophysical Research Letters, 38(4): L04601 https://doi.org/10.1029/2010GL046474
- Kleypas JA, McManus JW, Menez LAB. 1999. Environmental limits to coral reef development: Where do we draw the line? American Zoologist 39(1):146-159
- Sommer B, Beger M, Harrison PL, Babcock RC, Pandolfi JM. 2018. Differential response to abiotic stress controls species distributions at biogeographic transition zones. Ecography. 41(3):478-490
- Veron JEN, Minchin PR. 1992. Correlations between sea surface temperature, circulation patterns and the distribution of hermatypic corals of Japan. Continental Shelf Research. 12(7-8):835-857
- Buddemeier RW, Smith SV. 1999. Coral adaptation and acclimatization: A most ingenious paradox. American Zoologist. 39(1):1-9
- Riegl B, Piller WE. 1997. Distribution and environmental control of coral assemblages in northern Safaga Bay (Red Sea, Egypt). Facies. 36:141-162
- Beger M, Sommer B, Harrison PL, Smith SDA, Pandolfi JM. 2014. Conserving potential coral reef refuges at high latitudes. Diversity and Distributions. 20(3):245-257
- Harriott VJ, Banks SA. 2002. Latitudinal variation in coral communities in eastern Australia: a qualitative biophysical model of factors regulating coral reefs. Coral Reefs. 21(1):83-94
- Harriott VJ, Smith SDA, Harrison PL. 1994. Patterns of coral community structure of subtropical reefs in the Solitary Islands Marine Reserve, eastern Australia. Marine Ecology Progress Series. 109(1):67-76
- Guinotte JM, Buddemeier RW, Kleypas JA. 2003. Future coral reef habitat marginality: temporal and spatial effects of climate change in the Pacific basin. Coral Reefs. 22(4): 551-558
- Perry CT, Larcombe P. 2003. Marginal and non-reef-building coral environments. Coral Reefs. 22(4):427-432
- Harriott VJ, Harrison PL, Banks SA. 1995. The coral communities of Lord-Howe Island. Marine and Freshwater Research. 46(2):457-465
- Veron JEN, Done TJ. 1979. Corals and coral communities of Lord Howe Island. Australian Journal of Marine and Freshwater Research. 30(2):203-236
- Yamano H, Hori K, Yamauchi M, Yamagawa O, Ohmura A. 2001. Highest-latitude coral reef at Iki Island, Japan. Coral Reefs, 20(1):9-12
- Sommer B. 2015. Ecological dynamics of scleractinian corals at their high-latitude range margins. PhD Thesis. School of Biological Sciences. The University of Queensland. Brisbane, Australia. https://doi.org/10.14264/uql.2015.1083
- Wallace CC, Fellegara I, Muir PR, Harrison PL. 2009. The scleractinian corals of Moreton Bay, eastern Australia: High latitude, marginal assemblages with increasing species richness Memoirs of the Queensland Museum. 54(2):1-118
- Sommer B, Harrison PL, Beger M, Pandolfi JM. 2014. Trait-mediated environmental filtering drives assembly at biogeographic transition zones. Ecology. 95(4):1000-1009
- Veron JEN, How RA, Done TJ, Zell LD, Dodkin MJ, O’Farrell AF. 1974. Corals of the Solitary Islands, New South Wales. Marine and Freshwater Research. 25:193-208
- Lybolt M, Pandolfi JM. 2018. Holocene History of Moreton Bay Reef Habitats. In: Tibbetts IR, Hall NJ, and Dennison WC. (Eds). Moreton Bay Quandamooka & Catchment: Past, present, and future. School of Marine Sciences: Brisbane, The University of Queensland. Brisbane, Australia. Doi: 10.6084/m9.figshare.8072591
- Lovell ER. 1989. Coral assemblages of Moreton Bay, Queensland, Australia, before and after a major flood. Memoirs – Queensland Museum. 27:535-550
- Johnson PR, Neil DT. 1998. Susceptibility to flooding of two dominant coral taxa in Moreton Bay. In: Tibbetts IR, Hall NJ, and Dennison WC. (Eds). Moreton Bay Quandamooka & Catchment: Past, present, and future. School of Marine Sciences: Brisbane, The University of Queensland. Brisbane, Australia. p. 597-604
- Fellegara I. 2008. Ecophysiology of the marginal, high-latitude corals (Coelenterata : Scleractinia) of Moreton Bay, Qld. PhD Thesis. Centre for Marine Studies, The University of Queensland. Brisbane, Australia
- Sommer B, Sampayo EM, Beger M, Harrison PL, Babcock RC, Pandolfi JM. 2017. Local and regional controls of phylogenetic structure at the high-latitude range limits of corals. Proceedings of the Royal Society B-Biological Sciences. 284(1861):20170915 doi: 10.1098/rspb.2017.0915.
- Wallace CC. 1999. Staghorn corals of the world. Collingwood, Australia: CSIRO Publishing
- Veron JEN. 1993. A biogeographic database of hermatypic corals. Species of the Central Indo-Pacific genera of the world. Townsville, Australia: Australian Institute of Marine Science
- Darling ES, McClanahan TR, Cote IM. 2013. Life histories predict coral community disassembly under multiple stressors. Global Change Biology. 19(6):1930-1940
- Narayan YR, Pandolfi JM. 2010. Benthic foraminiferal assemblages from Moreton Bay, South-East Queensland, Australia: Applications in monitoring water and substrate quality in subtropical estuarine environments. Marine Pollution Bulletin. 60(11):2062-2078
- Palmieri V, 1976. Modern and relict foraminifera from the central Queensland shelf. Queensland Government Mining Journal. 77:406-436
- Palmieri V. 1976. Recent and Sub-recent Foraminifera from the Wynnum 1:25 000 Sheet Area, Moreton Bay, Queensland. Queensland Government Mining Journal. 77: 364-384
- Hallock P, Lidz BH, Cockey-Burkhard EM, Donnelly KB. 2003. Foraminifera as bioindicators in coral reef assessment and monitoring: The FORAM Index. Environmental Monitoring and Assessment. 81(1-3):221-238
- Lybolt M, Neil D, Zhao JX, Feng YX, Yu KF, Pandolfi JM. 2011. Instability in a marginal coral reef: the shift from natural variability to a human-dominated seascape. Frontiers in Ecology and the Environment. 9(3):154-160
- Moss A, Connell D, Bycroft B. 1992. Water quality in Moreton Bay. In: Crimp O. (Ed). Moreton Bay in the Balance. Australian Littoral Society. Brisbane, Australia
- Neil DT. 1998. Moreton Bay and its catchment: Seascape and landscape, development and degradation. In: Moreton Bay and Catchment, Tibbetts IR, Hall NJ, Dennison WC (Eds). The University of Queensland, School of Marine Sciences. Brisbane, Australia. 3-54
- Sen Gupta BK, Machain-Castillo ML. 1993. Benthic foraminifera in oxygen-poor habitats. Marine Micropaleontology. 20(3-4):183-201
- Hallock P. 2012. The FORAM Index revisited: uses, challenges and limitations. In: 12th International Coral Reef Symposium. Cairns, Australia: James Cook University
- Neil DT. 1993. The geomorphic significance of Green Island, Moreton Bay. In: Greenwood JG, Hall NJ (Eds). Future marine science in Moreton Bay. School of Marine Science, The University of Queensland. Brisbane, Australia. p. 149-150
- Barnes RSK, Barnes MKS. 2011. Hierarchical scales of spatial variation in the smaller surface and near-surface macrobenthos of a subtropical intertidal seagrass system in Moreton Bay, Queensland. Hydrobiologia. 673(1):169-178
- Skilleter GA. 1998. Ecology of benthic invertebrates in Moreton Bay. In: Tibbetts IR, Hall NJ, and Dennison WC. (Eds). Moreton Bay Quandamooka & Catchment: Past, present, and future. School of Marine Sciences: Brisbane, The University of Queensland. Brisbane, Australia. p. 365-394
- Hutchings P. 1999. Taxonomy of estuarine invertebrates in Australia. Australian Journal of Ecology. 24(4):381-394
- Rachello Dolmen P. 2013. Biodiversity and Historical Ecology of Marine Gastropod Assemblages from Subtropical Moreton Bay, Queensland, Australia. PhD Thesis. School of Biological Sciences. The University of Queensland: Brisbane, Australia. doi: https://doi.org/10.14264/uql.2014.95
- Davie PJF, Hooper JNA. 1998. Patterns of biodiversity in marine invertebrate and fish communities of Moreton Bay. In: Tibbetts IR, Hall NJ, and Dennison WC. (Eds). Moreton Bay Quandamooka & Catchment: Past, present, and future. School of Marine Sciences: Brisbane, The University of Queensland. Brisbane, Australia. p. 331-346
- Ridgway KR, Dunn JR. 2003. Mesoscale structure of the mean East Australian Current System and its relationship with topography. Progress in Oceanography. 56(2):189-222
- Lovell ER. 1976. The reef building corals (Coelenterata: Scleractinia) of Moreton Bay, Queensland: their distribution and ecology. Masters Thesis. The University of Queensland: Brisbane, Australia
- Wells JW. 1955. Recent and subfossil corals of Moreton Bay, Queensland. The University of Queensland Papers, Department of Geology. 4:3-23
- Stutchbury S. 1855. Geological and mineralogical surveys [of the Colony of New South Wales]: 12th, 13th and 14th reports. Sydney, Australia: Govt. Printer
- Saville-Kent W. 1893. The Great Barrier Reef of Australia: its products and potentialities. In: The Great Barrier Reef of Australia: its Products and Potentialities. p. 387
- Science Student Association. 1946. Report on the research expedition to Moreton Bay. The University of Queensland, Brisbane, Australia
- Pickett JW, Thompson CH, Kelley RA, Romans D 1985. Evidence of high sea-level during isotope stage – 5C in Queensland, Australia. Quaternary Research. 24(1):103-114
- Lybolt MJ. 2012. Dynamics of marginal coral reef ecosystems: Historical responses to climatic and anthropogenic change. PhD Thesis. School of Biological Sciences. The University of Queensland: Brisbane, Australia
- Narayan YR, Lybolt M, Zhao JX, Feng YX, Pandolfi JM. 2015. Holocene benthic foraminiferal assemblages indicate long-term marginality of reef habitats from Moreton Bay, Australia. Palaeogeography Palaeoclimatology Palaeoecology. 420:49-64
- Walters I. 1992. Antiquity of marine fishing in south-east Queensland. Queensland Archaeological Research. 9:35-37
- Walters I. 1989. Intensified fishery production at Moreton Bay, southeast Queensland, in the late Holocene. Antiquity. 63(239):215-224
- Walters I. 1992. Farmers and their fires, fishers and their fish – Production and productivity in pre-European south-east Queensland. Dialectical Anthropology. 17(2):167-182
- Hiscock P. 1994. Technological responses to risk to Holocene Australia. Journal of World Prehistory. 8(3):267-292
- Donders TH, Wagner F, Visscher H. 2006. Late Pleistocene and Holocene subtropical vegetation dynamics recorded in perched lake deposits on Fraser Island, Queensland, Australia. Palaeogeography Palaeoclimatology Palaeoecology. 241(3-4):417-439
- Neil DT, Orpin AR, Ridd EV, Yu BF. 2002. Sediment yield and impacts from river catchments to the Great Barrier Reef lagoon. Marine and Freshwater Research. 53(4):733-752
- McCulloch M, Fallon S, Wyndham T, Hendy E, Lough J, Barnes D. 2003. Coral record of increased sediment flux to the inner Great Barrier Reef since European settlement. Nature. 421(6924):727-730
- Walden W, Bycroft B. 1998. Non-point source pollutant estimation in Brisbane River and Moreton Bay, In: Tibbetts IR, Hall NJ, and Dennison WC. (Eds). Moreton Bay Quandamooka & Catchment: Past, present, and future. School of Marine Sciences: Brisbane, The University of Queensland. Brisbane, Australia. p. 229-238
- Allingham DP, Neil DT. 1995. The supratidal deposits and effects of coral dredging on Mud island, Moreton Bay, Southeast Queensland. Zeitschrift Fur Geomorphologie. 39(3):273-292
- Flood PG. 1978. The significance of two contrasting sedimentary environments (the fringing coral reef and the tidal mud flat) presently in juxtaposition along the southwestern shore of Moreton Bay, Queensland. University of Queensland Papers, Department of Geology. 8:44-65
- Schaffelke B, Mellors J, Duke NC. 2005. Water quality in the Great Barrier Reef region: responses of mangrove, seagrass and macroalgal communities. Marine Pollution Bulletin. 51(1-4):279-296
- Hopley D. 1982. The geomorphology of the Great Barrier Reef : Quaternary development of coral reefs. New York: Wiley
- Kittinger JN, Pandolfi JM, Blodgett JH, Hunt TL, Jiang H, Maly K, McClenachan LE, Schultz JK, Wilcox BA. 2011. Historical reconstruction reveals recovery in Hawaiian coral reefs. Plos One. 6(10): e25460
- Murray JW. 2007. Biodiversity of living benthic foraminifera: How many species are there? Marine Micropaleontology. 64:163-176
- Hallock P. 1985. Future farmers of the sea. Natural History. 94(3):60
- Reymond CE, Uthicke S, Pandolfi JM. 2012. Tropical foraminifera as indicators of water quality and temperature. In 12th International Coral Reef Symposium. Cairns, Australia: James Cook University
- Narayan YR. 2011. Benthic foraminifera as Holocene to Recent indicators in marginal marine environments: modern distribution and the palaeoecological response to environmental changes in Moreton Bay, southeastern Queensland, Australia, in School of Earth Sciences. The University of Queensland: Brisbane, Australia. 236
- Roche RC, Perry CT, Johnson KG, Sultana K, Smithers SG, Thompson AA. 2011. Mid-Holocene coral community data as baselines for understanding contemporary reef ecological states. Palaeogeography Palaeoclimatology Palaeoecology. 299(1-2):159-167
- Beesley PL, Ross GJB, Wells A. 1998. Mollusca: the Southern Synthesis. Part A and B. Fauna of Australia. Vol. 5. Melbourne, Australia: CSIRO Publishing
- Gray JS. 1979. Pollution-induced changes is populations. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 286(1015):545-561
- McGill BJ, Etienne RS, Gray JS, Alonso D, Anderson MJ, Benecha HK, Dornelas M, Enquist BJ, Green JL, He FL, Hurlbert AH, Magurran AE, Marquet PA, Maurer BA, Ostling A, Soykan CU, Ugland KI, White EP. 2007. Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Ecology Letters. 10(10):995-1015
- Tibbetts IR, Townsend KA. 2010. The abundance, biomass and size of macrograzers on reefs in Moreton Bay, Queensland. Memoirs of the Queensland Museum. 54(3):373-384
- Kawecki TJ. 2008. Adaptation to marginal habitats. pp. 321-342. In: Annual Review of Ecology Evolution and Systematics.
- Munday PL, Warner RR, Monro K, Pandolfi JM, Marshall, DJ. 2013. Predicting evolutionary responses to climate change in the sea. Ecology Letters. 16(12):1488-1500
- Costello MJ. 2014. Long live marine reserves: A review of experiences and benefits. Biological Conservation. 176:289-296
- Cooper TF, Gilmour JP, Fabricius KE. 2009. Bioindicators of changes in water quality on coral reefs: Review and recommendations for monitoring programmes. Coral Reefs. 28(3):589-606
- Werner SR, Spurgeon JPG, Isaksen GH, Smith JP, Springer NK, Gettleson DA, N’Guessan L, Dupont JM. 2014. Rapid prioritization of marine ecosystem services and ecosystem indicators. Marine Policy. 50:178-189
- Narayan GR, Westphal H. 2016. Are Zanzibar’s reefs undergoing ecological change? Foraminifera bio-indicators for monitoring and assessment of reef ecosystems in the Western Indian Ocean. in 13th International Coral Reef Symposium. Honolulu, USA: International Society for Reef Studies
- Schueth JD, Frank TD. 2008. Reef foraminifera as bioindicators of coral reef health: Low Isles Reef, northern Great Barrier Reef, Australia. Journal of Foraminiferal Research. 38(1):11-22
- Uthicke S, Thompson A, Schaffelke B. 2010. Effectiveness of benthic foraminiferal and coral assemblages as water quality indicators on inshore reefs of the Great Barrier Reef, Australia. Coral Reefs. 29(1):209-225
- Lewis SE, Wust RAJ, Webster JM, Shields GA. 2008. Mid-late holocene sea-level variability in eastern Australia. Terra Nova. 20(1):74-81
- Sloss CR, Murray-Wallace CV, Jones BG. 2007. Holocene sea-level change on the southeast coast of Australia: a review. Holocene. 17(7):999-1014
