-
Previous paper
Projected changes to population, climate, sea-level and ecosystems
Megan I. Saunders1,2, Rebecca Runting3, Elin Charles-Edwards2, Jozef Syktus4 and Javier Leon5 -
This paper
Primary producers in Moreton Bay: Phytoplankton, benthic microalgae and filamentous cyanobacteria
Saeck, Emily1,2, Grinham, Alistair3, Coates Marnane, Jack1, McAlister, Tony4, Burford, Michele*1 -
Next paper
Seagrasses of Moreton Bay <i>Quandamooka</i>: Diversity, ecology and resilience
Paul Maxwell1,7, Rod Connolly2, Chris Roelfsema3 Dana Burfeind4,5James Udy6 Kate O’Brien7 Megan Saunders7 Richard Barnes8, Andrew Olds9, Chris Hendersen9, Ben Gilby9
Primary producers in Moreton Bay: Phytoplankton, benthic microalgae and filamentous cyanobacteria
Authors
Saeck, Emily1,2, Grinham, Alistair3, Coates Marnane, Jack1, McAlister, Tony4, Burford, Michele*1Author affiliations
- Australian Rivers Institute, Griffith University, Nathan, Queensland 4111, Q, Australia
- Healthy Land & Water, Level 19, 160 Anne Street, Brisbane, 4000, Q, Australia
- School of Civil Engineering, University of Queensland, St Lucia 4072, Q, Australia
- Water Technology, Level 3, 43 Peel St, South Brisbane, 4104, Q, Australia
Corresponding author
e.saeck@griffith.edu.auORCID
Emily Saeck: https://orcid.org/0000-0002-8548-0300
Alistair Grinham: https://orcid.org/0000-0001-8313-2276
Jack Coates-Marnane: https://orcid.org/0000-0002-8418-4825
Tony McAlister: https://orcid.org/0000-0001-6802-2183
Michele Burford: https://orcid.org/0000-0002-1076-6144
Book
Primary producers in Moreton Bay: Phytoplankton, benthic microalgae and filamentous cyanobacteria
Chapter
Research Paper Title
Primary producers in Moreton Bay: Phytoplankton, benthic microalgae and filamentous cyanobacteria
Cite this paper as:
Saeck E, Grinham A, Coates-Marnane J, McAlister T, Burford M. 2019. Primary producers in Moreton Bay: Phytoplankton, benthic microalgae and filamentous cyanobacteria. In Tibbetts, I.R., Rothlisberg, P.C., Neil, D.T., Homburg, T.A., Brewer, D.T., & Arthington, A.H. (Editors). Moreton Bay Quandamooka & Catchment: Past, present, and future. The Moreton Bay Foundation. Brisbane, Australia. Available from: https://moretonbayfoundation.org/
DOI
10.6084/m9.figshare.8074355
ISBN
978-0-6486690-0-5
Abstract
Phytoplankton and benthic microalgae are critical to the ecosystem productivity of Moreton Bay. The Bay is oligotrophic for most of the year, with acute nutrient pulses delivered by high rainfall events. These nutrient pulses are important drivers of primary production leading to phytoplankton growth and shifts in species composition. Consistent with many coastal areas of the world, the phytoplankton community is dominated by diatoms and a range of pico- and nanoplankton. A west to east gradient of phytoplankton standing stocks across the Bay reflects the influence of river and groundwater discharges. In the past, sewage discharge has also been a significant driver of phytoplankton growth in the western region of the Bay, particularly prior to 2000 when a sewage treatment plant at the mouth of the Brisbane River was upgraded to reduce nutrient discharges. The management of sewage successfully reduced phytoplankton standing stocks, and appears to have improved resilience to acute rainfall events. Acute rainfall events also deliver pulses of sediments, particularly from catchments cleared of vegetation, which affects Moreton Bay light conditions in the water column and silt content of the sediments. The species composition of benthic microalgae (BMA) in the Bay is dominated by diatoms and is driven by the silt content of the sediment. It is hypothesised that low light conditions limit benthic algae and phytoplankton productivity during and following events, in the same way that light limitation affects seagrass productivity; however, research in this area is limited. The exception to diatom dominance in the shallow sediments is in locations where the toxic benthic cyanobacterium Lyngbya majuscula occurs. L. majuscula blooms have become regular in occurrence, especially in the north western Bay. Anthropogenic influences including changes in nutrient inputs likely led to these increased occurrences. Overall the phytoplankton and BMA biomass and species composition of the Bay reflect a relatively healthy system that has improved in response to management intervention. Despite this, persistent chronic pressure from catchment-derived sediment and nutrients has potential to erode this resilience.
Keywords: microphytobenthos, diatoms, flood, cyanobacteria, productivity, sewage, Lyngbya, nutrient limitation
Background
Phytoplankton and benthic microalgae (BMA) are critical for the productivity, water quality, habitat condition and biodiversity in Moreton Bay (the Bay). In the Bay it is estimated that phytoplankton contribute 74% to Bay productivity and BMA 9% (5). In coastal systems globally, these primary producers are under pressure from increasing sediment and nutrient enrichment caused by human development. Nutrient enrichment can cause persistent phytoplankton blooms and shifts in phytoplankton community composition, which in extreme cases leads to harmful algal blooms (HABs) and hypoxic dead zones (6–8). Such conditions in turn affect light and oxygen at the benthic interface, leading to species shifts and growth limitation of the benthic microalgae (BMA). Changes in and loss of BMA affect nutrient cycling and increases nutrient availability for pelagic productivity, furthering degrading water quality and habitat conditions (5, 9).
Moreton Bay is naturally oligotrophic—low nutrients, low productivity (<100 g C m-2 y-1 following the Nixon (10) classification)—and on a global scale it is a relatively undisturbed system (11–13). However southeast Queensland is one of the fastest growing regions in Australia, with a current human population of 3.5 million (14) and over the years there have been indications of anthropogenic impacts, specifically eutrophication, in some regions of the Bay. This chapter synthesises the existing understanding of phytoplankton and BMA communities of the Bay, and highlights human pressures that may impact growth and species composition of these primary producers.

Phytoplankton
Community characteristics
Abundance and distribution
Moreton Bay is oligotrophic for most of the year with acute nutrient pulses delivered by high rainfall events that stimulate productivity (15–18). These nutrient pulses are important drivers of primary production leading to phytoplankton growth (19–21). A west to east gradient of phytoplankton standing stocks across the Bay reflects the influence of river discharges (Fig. 1). Overall the mean annual chlorophyll (Chl a) concentration in the Bay is 2.09±0.5 µg L-1, based on monthly samples between 2006-2016 at 67 long-term monitoring sites (2) (Table 1). In the western and southern regions of the Bay, there is a significant riverine influence, with mean annual Chl a concentration of 2.20 ±0.7 and 2.36 ±0.8 µg L-1 respectively. The range of annual maximum Chl a for these regions is 6.2-37.4 and 7.3-44 µg L-1 respectively. In the eastern bay Chl a levels are relatively low, as this region is least influenced by river discharges and has the strongest oceanic influence (0.87±0.28 µg L-1). Chl a concentrations are highest during the wet summer months when rainfall and runoff is highest (approximately September-April), and lowest in the dry winter months (approximately May to August) (12, 22, 23).
Queensland Government policy sets water quality objectives for maintaining the environmental value of the Bay, which include objectives for Chl a concentrations. The mean annual Chl a concentrations in the western and southern regions for the period 2006 – 2016 reached or slightly exceeded these guideline values. In contrast, the eastern regions more frequently fall below guideline maximum values (Saeck et al. this volume (25)) (Table 1).
Table 1. The Mean chlorophyll a concentrations (µg L-1) in Moreton Bay based on long-term monitoring sites, compared against the Queensland Government (2009) water quality objectives for different regions of the Bay (Ecosystem Health Monitoring Program (EHMP)) sampled monthly for the period 2006-2016 (n = approx. 120 per site) (2, 24).
| Region | Mean annual Chl a (µg L-1) (±SD) | Min (µg Chl a L-1) | Max (µg Chl a L-1) | Queensland Govt water quality objectives (2009) (µg Chl a L-1) (21) |
| Moreton Bay overall | 2.09±0.5 | <0.10 | 44.0 | |
| Western bay | 2.20 ±0.7 | <0.10 | 37.4 | <2.0 |
| Southern bay | 2.36 ±0.8 | <0.10 | 44.0 | <2.0 |
| Eastern bay | 0.87±0.28 | <0.10 | 11.1 | <1.0 |
Productivity rates
In 1997, O’Donohue & Dennison (26) concluded that Moreton Bay has overall low areal phytoplankton productivity (<25 mg C m-2 h-1) due to light and nitrogen limitation during summer and temperature limitation during winter (26, 27). Peaks in phytoplankton productivity occur following rainfall events. The system is slightly net autotrophic, with an estimated 3% of carbon being exported to the ocean. It is hypothesised that rapid recycling of the nitrogen pool in the water column supports these rates of primary productivity within the Bay (5, 28).

Few studies have measured phytoplankton productivity rates in Moreton Bay since 2000 (5, 23, 29). Saeck (unpublished data) measured productivity rates across the Bay in 2009 and 2010 using 13C uptake incubations as per Burford et al. (30). The study found areal productivity rates significantly higher in the delta region of southern Moreton Bay compared with the central region (Fig. 2). Cloern (13) reviewed phytoplankton productivity rates across natural and modified coastal systems globally and reported that 29 mg C m-2 h-1 is the median rate, with maximum rates of 215 mg C m-2 h-1. As such, in a global context, measured Moreton Bay rates fall below global medians, especially during dry conditions (noting that methods to measure productivity differs between studies and can cause significant variation in rates) (Fig. 2) (13).
Community composition
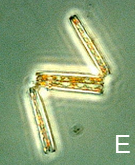
The Moreton Bay phytoplankton community includes species most typical of the temperate neritic assemblage, as described by Jeffrey & Hallegraeff (31), with an abundance of chain-forming diatoms (Fig. 3, A-D) and a low proportion of nano- and picoplankton (1, 32, 33). The community responds to highly variable and episodic intrusion of land-derived nutrients, following which the ever-present diatom populations form peaks in biomass (1). However, there is spatial variation across the Bay, with relatively more oceanic and dinoflagellate species in the northern regions compared with the south (29, 32, 34).
Figure 3: Images of phytoplankton sampled from Moreton Bay (29): A) Skeletonema costatum; B) Asterionellopsis sp.; C) Chaetoceros sp.; D) Protoperidinium sp., and E) Thalassionema sp.
Nutrient status
Moreton Bay phytoplankton growth rates are typically limited by nitrogen, meaning that when nitrogen availability increases growth is stimulated. This observation is based on: coupled physical and biogeochemical modelling of Moreton Bay (35, 36); phytoplankton bioassay experiments (23, 26, 29, 37); and trend analyses of water quality monitoring data (22). Nutrient budgets calculated for Moreton Bay have found the rivers and catchment only contribute 1% of phytoplankton demand for dissolved inorganic nitrogen, suggesting significant reliance on nitrogen recycling, benthic fluxes and N fixation to meet nitrogen demands (5).
The degree of nitrogen limitation varies across the Bay, reflecting the west to east nutrient gradient and the influence of river discharges and groundwater input (38). Specifically, studies have found low or no phytoplankton response (measured as growth, carbon uptake and/or photosynthetic yield, Fv/Fm) to nitrogen in samples collected from the nearshore areas at the mouths of Brisbane and Logan River, whereas samples from the central to eastern Bay consistently responded to nitrogen addition (29, 37, 39).
Furthermore, Saeck et al. (37) found that when ambient dissolved inorganic nitrogen concentrations were higher than 2 µm L-1, phytoplankton photo-synthetic yield (Fv/Fm) in bioassays of Moreton Bay water samples did not respond to additions of dissolved nitrogen (N). This suggests an ecological threshold above which Moreton Bay phytoplankton consistently have a high potential for growth and subsequent blooms, unless limited by other factors such as light, Phosphorus (P), silica (S), iron (Fe) or residence times.
In terms of nutrients other than N, Glibert et al. (23), O’Donohue et al. (26) and Quigg et al. (29) found little to no increase in growth response with P additions. Phytoplankton may respond to P by increased P storage, rather than growth. However, the lack of an increased response to N and P together suggests that there is no co-limitation of these two nutrients. Glibert et al. (23) similarly found minimal response to Si, and concluded that Si is unlikely to limit phytoplankton biomass in the Bay.
Pressures
Vulnerability to acute nutrient inputs from high inflow events
Increased phytoplankton abundance following high rainfall and river inflow events is a pattern broadly found in coastal studies (e.g. Burford et al. (40); Valdes-Weaver et al. (41)). Runoff events deliver new dissolved inorganic nitrogen and Moreton Bay phytoplankton respond by increasing productivity, growth rates and photosynthetic yields (1, 23, 26). Typically, there are increased abundance of phytoplankton in the western and southern regions of Moreton Bay following events, irrespective of season (1, 29, 32).
Phytoplankton community composition varies when comparing different high inflow events, with no consistent pattern of response. This is because the conditions associated with each event are unique, as every flood varies in nutrient, turbidity and flow characteristics. Previous studies of coastal systems have reported significant variability in species response patterns to new nutrient inputs, not only between locations but also between events (42, 43). In Bramble Bay, Saeck (1) found diatoms consistently dominated the initial peak — typically large chain-forming species — but the proportion of these species varied. In the 1996 Brisbane River flood event the community was dominated by Bacillariaceae and Rhizosoleniaceae; the 2009 event was diverse with significant contributions from Chaetocerotaceae, Thalassiosiraceae, Fragilariaceae and Leptocylindraceae; while the 2011 event was dominated by Skeletonemaceae and Chaetocerotaceae (1, 44).
Phytoplankton blooms off the east coast of Australia have been described by Hallegraeff and Jeffrey (45) to follow a predictable succession pattern from dominance by small chain-forming species to large centric species and eventually to large dinoflagellates. Most studies of the Bay have not detected a consistent diatom to dinoflagellate succession pattern following high flow events (1, 44){Heil, 1998 #123;Heil, 1998 #123}, but this may be due to the limited sampling frequency following events.
In addition to nutrients, high flow events introduce suspended particulates that strongly increase light attenuation and would be expected to influence pelagic primary productivity. Light limitation affects BMA and seagrass productivity in the Bay (46–48). As such, by extension, it is hypothesised that light limitation would act on phytoplankton productivity following events. However, research in this area is limited.
Vulnerability to chronic sewage nutrient inputs
Studies have shown that discharged sewage loads discharged can impact the phytoplankton community dynamics, including increasing standing stocks and extending the duration of blooms resulting from catchment flow events (9, 49, 50). Historically (prior to 2000) Bramble Bay, in western Moreton Bay, was characterised by elevated nitrogen concentrations (4 – 8 μmol L-1 dissolved inorganic nitrogen (DIN)) and phytoplankton biomass (4 – 10 μg L-1 Chl a) (22). This eutrophication was found to result from sewage related nitrogen (22, 35, 36, 51) and prompted major investments to improve the nitrogen removal capacity of sewage-treatment plants in the region. The reductions in chronic sewage nitrogen loads resulted in reduced mean monthly Chl a concentrations. Following sewage reductions (post 2003) Chl a concentrations were consistently lower at inshore sites, compared with years prior to the sewage treatment plant upgrades (mean below 2.0 and 4.5 μgL-1 for Stations 921 and 906 respectively) (2, 22) Saeck et al., this volume (25).

Comparison of the response of phytoplankton to the high flow events of 1996 (prior to sewage N reductions) and 2011 (decade after sewage N reductions), found that the two events were associated with a total annual nitrogen load of similar size. However, the phytoplankton response to these events was significantly different (Fig. 4) (22). A single runoff event can deliver a nutrient load larger than the total annual sewage treatment plant (STP) load. Notably, the bloom associated with the 2011 flood peaked and fell within two weeks of event, compared with the 1996 flood when high phytoplankton abundance persisted throughout the year (Fig. 4). This suggests that reduction of chronic nutrient loads to Moreton Bay may have been successful in improving system resilience to these large nutrient loading events.
Moreton Bay, like other sub-tropical and tropical coastal ecosystems, is particularly vulnerable to shifts in nutrient delivery patterns (i.e. from acute events to chronic loading) across all seasons. This is because temperature infrequently falls low enough between seasons to limit growth (39). In comparison, in temperate estuaries, sewage nutrients have been found to affect phytoplankton Chl-a only during the warmer summer months (50).
Benthic microalgae (BMA)
Community characteristics
Role in sediment nutrient flux to surface waters
Benthic microalgae (BMA) are found at the sediment water interface (52). Their community composition largely mirrors that of phytoplankton with all major algal groups represented including cyanobacteria, diatoms, dinoflagellates and chlorophytes (53, 54). BMA play a key role in nutrient cycling, occupying the zone between anoxic sediment porewaters and oxic surface waters (5, 55). Sediment porewaters in the Bay generally have two to three orders of magnitude higher of DIN and P concentrations compared to overlying surface waters (36). Oxygenation of sediment surface layers through BMA photosynthesis controls the flux of available N and P to the surface waters (56, 57). However, blooms of N-fixing benthic cyanobacteria can fix significant amounts of N and increase N availability to surface waters (58, 59). The relative importance of N fixation to the overall nutrient budget is dependent upon the frequency and magnitude of these blooms (60).
Abundance and distribution

Udy et al. (3) studied the biomass and distribution of BMA at 55 sites across the Bay and found significant patchiness across the Bay, reporting high variability within and between sites. The highest densities were found on the intertidal banks and at depths shallower than 5 m (Fig. 5). Biomass, as indicated by Chl a concentration, ranged from 0 to 195 mg Chl a m-2. Seasonal patterns in Chl a appear to differ between the western and eastern Bay. Specifically, during the cool dry season BMA Chl a increases in the western Bay and this is hypothesised to be the result of improved water quality conditions during this period. In contrast, in the eastern Bay, where water quality is higher year round, BMA Chl a declines during winter.
Productivity rates
Studies show that the productivity of BMA in the Bay is strongly seasonal, with significant influence of temperature and light availability. Grinham et al. (47) reported that BMA productivity across the Bay was typically higher in summer than winter. During summer, when temperatures were highly suitable for growth, productivity was primarily influenced by light and, consequently, by water clarity.
In shallow coastal systems, BMA can contribute up to 50% of the total primary productivity (61), although in Moreton Bay, BMA productivity is estimated to be 9% of total carbon inputs (5). The overall productivity of BMA across the Bay has been estimated in several studies and these range from 50 to 350 mg C m-2 d-1 (5, 28, 36, 47). As such, BMA and phytoplankton represents the smallest biomass in the Bay compared with seagrass and mangrove (i.e. <1% of the total carbon). However, they contribute the highest productivity (producing 81% of total C y-1) (3, 5, 36). This supports the theory that they have significant influences on nutrient and sediment processes across the Bay.
Community composition
The Moreton Bay BMA community is dominated by pennate diatoms (3, 47, 62), a pattern typical of many other temperate and tropical coastal systems (52, 53). BMA species typically found in the Bay include those of genera: Pleurosigma, Navicula, Achanthes, Cocconies, Cyclotella, Paralia, Grammatophora, Amphora, and Dimmeregramma (3, 47, 62). Like water column phytoplankton, there is spatial variation across the Bay in terms of species composition.
Benthic diatoms, and other BMA, live on top of (epipelic) or attached to (epipsammic) the sediment particles. Moreton Bay benthic diatom assemblages are predominantly epipsammic, due to the presence of tidal sand banks made up of terrestrial and marine sands (54). However, there is increasing silt content in the southern areas of the Bay, and this shifts the diatom community structure, increasing the epipelic fraction. It also increases the overall species diversity and favours larger species (47, 51) in these areas. This silt effect has been found in other studies (63– 65).
Pressures
Vulnerability to chronic terrestrial sediment and nutrient inputs
Catchment development in South East Queensland has elevated sediment and nutrient loads delivered to Moreton Bay. Consequently, in the western and southern regions of the Bay nutrient concentrations, silt content and light attenuation are all higher (66). This has affected the productivity, and community composition of Moreton Bay BMA communities, which negatively affects benthic nutrient assimilation capacity reducing benthic productivity more than 50% compared to pre-European settlement (67).
Elevated nutrient concentrations have the potential to increase benthic productivity under high light conditions (47). However, nutrients also stimulate phytoplankton biomass, which can limit benthic light availability. Grinham et al. (47) found that productivity was not significantly higher in western and southern Moreton Bay even though nutrient concentrations were relatively higher (68). It was concluded this was the result of light limitation, caused by elevated phytoplankton and suspended sediment levels. Overall, reduced water clarity narrows the depth in which BMA grow and reduces the productivity of those areas.

Experimental studies in other systems demonstrate that BMA species composition may shift in response to nutrient enrichment (64, 69, 70). Sediment cores suggest that in central Moreton Bay, the diatom community as a whole (benthic and planktonic species) has undergone significant changes following the onset of increased nutrient and sediment yields of the coastal rivers draining into the Bay (71). Most notably, there has been a decrease in the abundance of typically benthic diatoms (Pleurosigma fenestrata, Cylotella litoralis, Grammataphora, Dimmeregramma) coupled with an increase in the occurrence of planktonic marine diatoms (Thalassiosira, Thalassiothrix) and chain forming species (i.e. Chaetoceros, Ceratulina bicornis). This was also apparent during the 2011 flood event where planktonic diatoms were stimulated over benthic forms (Fig. 6).
Grinham et al. (54) found increased diatom diversity associated with silt content across Moreton Bay. Fine sediments are accumulating in the western Bay (73). However, the impact of muddy sediments on BMA community composition has not been studied. Coates-Marnane et al. (73) predicted that the fine sediment accumulation in Moreton Bay may be approaching a threshold beyond which sediment resuspension will accelerate and cause chronic light limitation. Generally, in coastal systems, under extreme light attenuation the structure of BMA communities can undergo large shifts, with biodiversity declining until they are completely lost from the system (10). This may have significant implications for nutrient cycling and water quality, and on populations that depend on BMA communities as a source of nutrition, including some benthic invertebrates and herbivorous fishes.
Vulnerability to Lyngbya majuscula blooms
Intertidal and subtidal areas of Moreton Bay are vulnerable to blooms of the toxicfilamentous cyanobacterium Lyngbya majuscula. This N-fixing and toxin-producing cyanobacteria occurs naturally in the Bay, growing on sediment or attached to macroflora, such as seagrass. Outbreaks of very high biomass can occur when trace nutrients (e.g. Fe) from surface and ground water are available and light and temperature conditions are favourable (59, 74–76). These harmful algal blooms have been occurring in Moreton Bay since 1997, although reports suggest episodic blooms occurred periodically prior to this date, but not at the same scale or frequency (Fig. 7) (77). The increasing occurrences of blooms can be linked to increased nutrient loading. However, the relationship between nutrients and blooms is highly complex and environmental conditions, such as light, temperature, current velocities and redox state of the sediments, must also be favourable (78).

The impact of Lyngbya on BMA productivity, distribution and community composition has not been investigated. Lyngbya blooms affect light, nutrient and oxygen availability at the benthos (79), which is likely to have a significant impact to the BMA. Studies have found Lyngbya blooms can cause shifts in the meiofaunal species assemblages and their depth distribution in the sediments (79, 80). Similarly shifts in the BMA community composition would be expected.
Conclusion
Phytoplankton and BMA are critical to the productivity of Moreton Bay and are key indicators of water quality, habitat condition and biodiversity. The Bay remains predominantly oligotrophic with peaks in growth and productivity stimulated by acute nutrient pulses delivered by high rainfall events. Compared to coastal systems around the world, the rates of productivity and abundance of these primary producers are relatively low. This suggests that, despite pressure from human development, the Bay remains relatively healthy and resilient to current levels of nutrient and sediment input from activities within the catchment. Extensive monitoring and management, specifically the investment in upgrades of sewage treatment plants in the early 2000s, have contributed to significant improvements and protection of this resilience.
The Bay’s phytoplankton community appears to be resilient to both long-term and short-term changes in nutrient inputs, with no evidence of permanent state shifts to date in response to such changes. Historical trends show that persistently elevated phytoplankton abundance was associated with elevated N concentrations related to sewage nutrient inputs (pre-2003). This appears to have been a temporary change in the community rather than a permanent state shift. The trend was reversed when N in sewage discharges was lowered. This suggests that there was resilience over the long-term to chronic nutrient loads and that management was appropriate. Similarly, on the short-term seasonal scale, phytoplankton abundance and community composition shifts in response to acute nutrient inputs associated with large episodic rainfall events that are typical of sub-tropical and tropical systems. These shifts are also temporary, and the communities return to baseline conditions within approximately two weeks of an event.
The resilience of the Bay’s BMA community is less well understood. However, the communities appear to be relatively healthy. Exposure to short-term spikes in sediment loads associated with large episodic rainfall events may cause light limitation at depth and temporarily restrict BMA gross productivity. However, this is a natural and temporary response. More significant is the pressure from chronic sediment loads, with community shifts observed in places exposed to higher levels of siltation. There is a trend of lower BMA biomass in central Moreton Bay, an area of high ‘mud content’ associated with the Brisbane River plume, and in southern Moreton Bay. Over time high levels of siltation can result in loss of BMA from the system, a state change that would significantly limit Moreton Bay’s productivity and nutrient assimilation capacity.
Research on groundwater influences to Moreton Bay indicate that it is a major contributor to the hydrological and biogeochemical cycles, relative to riverine inputs (38). As such, the role of groundwater in driving productivity relative to other nutrient inputs may be significant, however, there is a gap in research in this area. There are also gaps in our understanding of light limitation impacts on phytoplankton and BMA communities, despite research suggesting sediment, which impacts light conditions, is considered a dominant pressure on Moreton Bay (81, 82).
Diffuse nutrient source management is a priority to protect the Bay from the current and growing pressure of siltation and eutrophication. Grinham et al. (83) demonstrated that nutrient concentrations and sediment discharge associated with major flood events are even higher than previously thought. As the Bay’s catchments remain degraded nutrient loads will continue to increase, as will infilling by sediment (73, 81, 82). These factors will increasingly impact biogeochemical processes and hence primary producers of the Bay. Catchment rehabilitation programs will have the twin benefits of reducing diffuse nutrient loading as well fine sediment particle deposition to the Bay. The reduction in fine sediments would also improve benthic light flux regime within the Bay and allow further reductions in nutrient availability by reducing the sediment nutrient flux contribution.
Maintaining long-term water quality monitoring is also a priority for tracking and responding to shifts in eutrophication pressure on the Bay. Over the years, monitoring of nutrient indicators in the Bay has been instrumental in the identifying and communicating the need for investment in nutrient management (84). However, nutrient pools are dynamic, with regeneration and update occurring at times scales not picked up with monthly monitoring. Nutrient concentrations and loads alone are not useful for predicting ecosystem effects (37). Monitoring programs that couple water quality indicators with ecosystem indicators, such as phytoplankton and BMA community composition and nutrient response, are critical for identifying pressures on the Bay ecosystems.
The conceptual diagrams in Figures 8 and 9 synthesise the existing understanding of phytoplankton and BMA communities of the Bay, and highlight how acute and chronic pressures from nutrient and sediment pollution may affect growth and species composition of these primary producers. While current trends and patterns suggest ecosystem resilience to such pressures, there is evidence that without action, ongoing chronic pressures could threaten and tip this resilience in Moreton Bay.


References
- Saeck EA. 2012. Nutrient dynamics of coastal phytoplankton: The role of episodic flow events and chronic sewage discharges. Griffith University. Brisbane
- EHMP. 2017. Ecosystem health monitoring program dataset. In: Healthy Land and Water, editor. Brisbane. hlw.org.au
- Udy JW, Dennison WC, Rogers J, Chaston K, Prange J, Duffy E, Duke NC. 1999. Task benthic flora nutrient dynamics (bfnd) – phase 2 final report. South East Queensland Regional Water Quality Management Strategy. Brisbane.
- South East Queensland Catchments. 2015. Seq natural resource management plan part three: State of the assets atlas. Brisbane: Ltd SEQC.
- Ferguson A, Eyre B. 2010. Carbon and N cycling in a shallow productive sub-tropical coastal emBayment (western moreton Bay, australia): The importance of pelagic–benthic coupling. Ecosystems. 13(7):1127-1144
- Cloern JE. 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series. 210:223-253
- Rabalais NN, Diaz RJ, Levin LA, Turner RE, Gilbert D, Zhang J. 2010. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences. 7(2):585-619
- Diaz RJ, Rosenberg R. 2008. Spreading dead zones and consequences for marine ecosystems. Science. 321(5891):926-929. 10.1126/science.1156401. http://www.sciencemag.org/cgi/content/abstract/321/5891/926
- Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, Brush G, Cornwell JC, Fisher TR, Glibert PM, Hagy JD, Harding LW, Houde ED, Kimmel DG, Miller WD, Newell RIE, Roman MR, Smith EM, Stevenson JC. 2005. Eutrophication of chesapeake Bay: Historical trends and ecological interactions. Marine Ecology-Progress Series. 303:1-29. <Go to ISI>://000234214500001
- Nixon SW. 1995. Coastal marine eutrophication – a definition, social causes, and future concerns. Ophelia. 41:199-219. <Go to ISI>://A1995QQ83600010
- Cloern JE, Jassby AD. 2010. Patterns and scales of phytoplankton variability in estuarine-coastal ecosystems. Estuaries and Coasts. 33(2):230-241
- Eyre B, Ferguson A, Webb A, Maher D, Oakes J. 2011. Metabolism of different benthic habitats and their contribution to the carbon budget of a shallow oligotrophic sub-tropical coastal system (southern moreton Bay, australia). Biogeochemistry. 102(1-3):87-110
- Cloern J, Foster S, Kleckner A. 2014. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences. 11(9):2477-2501
- The State of Queensland. 2017. Shapingseq south east queensland regional plan 2017. Brisbane: Department of Infrastructure LGaP.
- Eyre B. 2000. Regional evaluation of nutrient transformation and phytoplankton growth in nine river-dominated sub-tropical east australian estuaries. Marine Ecology Progress Series. 205:61-83
- Eyre BD, Ferguson AJP. 2006. Impact of a flood event on benthic and pelagic coupling in a sub-tropical east australian estuary (brunswick). Estuarine Coastal and Shelf Science. 66(1-2):111-122. <Go to ISI>://000234792800011
- Eyre BD, Pont D. 2003. Intra- and inter-annual variability in the different forms of diffuse N and phosphorus delivered to even sub-tropical east australian estuaries. Estuarine, Coastal and Shelf Science 57:137–148
- Junk WJ, Bayley PB, Sparks RE. 1989. The flood pulse concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106:110-127
- Mallin MA, Paerl HW, Rudek J, Bates PW. 1993. Regulation of estuarine primary production by watershed rainfall and river flow. Marine Ecology Progress Series. 93:199-203
- Loneragan NR, Bunn SE. 1999. River flows and estuarine ecosystems: Implications for coastal fisheries from a review and a case study of the logan river, southeast queensland. Australian Journal of Ecology. 24:431-440
- Gillanders BM, Kingsford MJ. 2002. Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanography and Marine Biology: an Annual Review. 40:233-309
- Saeck EA, O’Brien KR, Weber TR, Burford MA. 2013. Changes to chronic N loading from sewage discharges modify standing stocks of coastal phytoplankton. Marine pollution bulletin. 71(1):159-167
- Glibert PM, Heil CA, O’Neil JM, Dennison WC, O’Donohue MJH. 2006. N, phosphorus, silica, and carbon in moreton Bay, queensland, australia: Differential limitation of phytoplankton biomass and production. Estuaries and Coasts. 29(2):209-221. <Go to ISI>://000238346900004
- Department of Environment and Heritage Protection. 2009. Queensland water quality guidelines. Queensland Government. Brisbane. ISBN 978-0-9806986-0-2.
- Saeck E, Udy J, Maxwell P, Grinham A, Moffatt D, Senthikumar S, Udy D, Weber T. 2019. Water quality in moreton Bay and its major estuaries: Change over two decades (2000-2018). In: Tibbetts IR, P.; Neil, D.; Homburg, T.; Brewer, D.; Arthington, A. . (Ed.) Moreton Bay quandamooka & catchment: Past, present and future. The Moreton Bay Foundation, Brisbane, Australiahttps://moretonBayfoundation.org/
- O’Donohue MJH, Dennison WC. 1997. Phytoplankton productivity response to nutrient concentrations, light availability and temperature along an australian estuarine gradient. Estuaries. 20(3):521-533. <Go to ISI>://A1997XT91000005
- Eyre B, Mckee LJ. 1999. Task nutrient budgets (nb) phase 2 final report. South East Queensland Regional Water Quality Strategy. Brisbane.
- Eyre BD, Mckee LJ. 2002. Carbon, N, and phosphorus budgets for a shallow subtropical coastal emBayment (moreton Bay, australia). Limnology and Oceanography. 47(4):1043-1055
- Quigg A, Litherland S, Phillips JA, Kevekordes K. 2010. Phytoplankton productivity across moreton Bay, australia: The impact of water quality, light and nutrients on spatial patterns. . In: Davie PJF, Phillips JA. (Eds.) Proceedings of the thirteenth international marine biological workshop, the marine fauna and flora of moreton Bay, queensland Memoirs of the Queensland Museum – Nature (54), Brisbane, Australia. p. 355-372
- Burford M, Revill A, Palmer D, Clementson L, Robson B, Webster I. 2011. River regulation alters drivers of primary productivity along a tropical river-estuary system. Marine and Freshwater Research. 62(2):141-151
- Jeffrey SW, Hallegraeff GM. 1990. Phytoplankton ecology of australasian waters. In: Clayton MN, King RJ. (Eds.) Biology of marine plants. Longman Cheshire, Melbourne. p. 311-348
- Heil C, O’Donohue M, Dennison W. 1998. Aspects of the winter phytoplankton community of moreton Bay. In: Tibbetts I, Hall N, Dennison W. (Eds.) Moreton Bay and catchments. School of Marine Science, University of Queensland, Brisbane. p. 291-300
- Davies CH, Coughlan A, Hallegraeff G, Ajani P, Armbrecht L, Atkins N, Bonham P, Brett S, Brinkman R, Burford M. 2016. A database of marine phytoplankton abundance, biomass and species composition in australian waters. Scientific data. 3
- Saeck EA. 2011. Unpublished data.
- McEwan J, Gabric AJ, Bell PRF. 1998. Water quality and phytoplankton dynamics in moreton Bay, southeastern queensland. Ii. Mathematical modelling. Marine and Freshwater Research. 49(3):227-239. <Go to ISI>://000074990200004
- Dennison W, Abal E. 1999. Moreton Bay study: A scientific basis for the healthy waterways campaign. South East Queensland Regional Water Quality Management Strategy, Brisbane. pp. 246
- Saeck EA, Brien KRO, Burford MA. 2016. N response of natural phytoplankton communities: A new indicator based on photosynthetic efficiency fv/fm. Marine Ecology Progress Series. 552:81-92
- Stewart BT, Santos IR, Tait DR, Macklin PA, Maher DT. 2015. Submarine groundwater discharge and associated fluxes of alkalinity and dissolved carbon into moreton Bay (australia) estimated via radium isotopes. Marine Chemistry. 174:1-12
- O’Donohue MJ, Glibert PM, Dennison WC. 2000. Utilization of N and carbon by phytoplankton in moreton Bay, australia. Marine and Freshwater Research. 51(7):703-712. <Go to ISI>://000088943200007
- Burford MA, Rothlisberg PC, Wang YG. 1995. Spatial and temporal distribution of tropical phytoplankton species and biomass in the gulf of carpentaria, australia. Marine Ecology-Progress Series. 118(1-3):255-266. <Go to ISI>://A1995QP42600024
- Valdes-Weaver LM, Piehler MF, Pinckney JL, Howe KE, Rossignol K, Paerl HW. 2006. Long-term temporal and spatial trends in phytoplankton biomass and class-level taxonomic composition in the hydrologically variable neuse-pamlico estuarine continuum, north carolina, USA. Limnology and Oceanography. 51(3):1410-1420. <Go to ISI>://000237748300018
- Hallegraeff GM, Reid DD. 1986. Phytoplankton species succesions and their hydrological environment at a coastal station off sydney. Australian Journal of Marine and Freshwater Research. 37(3):361-377. <Go to ISI>://A1986C817600007
- Reynolds CS. 1984. Phytoplankton periodicity: The interactions of form, function and environmental variability. Freshwater Biology. 14(2)
- Heil C, O’Donohue M, Miller C, Dennison W. 1998. Phyotplankton community response to a flood event. In: Tibbetts I, Hall N, Dennison W. (Eds.) Moreton Bay and catchments. School of Marine Science, University of Queensland, Brisbane. p. 569-584
- Hallegraeff G, Jeffrey SW. 1993. Annually recurrent diatom blooms in spring along the new south wales coast of australia. Australian Journal of Marine and Freshwater Research. 44(2):325-334
- Maxwell PS, Pitt KA, Burfeind DD, Olds AD, Babcock RC, Connolly RM. 2014. Phenotypic plasticity promotes persistence following severe events: Physiological and morphological responses of seagrass to flooding. Journal of Ecology. 102(1):54-64
- Grinham AR, Carruthers TJ, Fisher PL, Udy JW, Dennison WC. 2007. Accurately measuring the abundance of benthic microalgae in spatially variable habitats. Limnology and Oceanography: Methods. 5(5):119-125
- Longstaff B, Dennison W. 1999. Seagrass survival during pulsed turbidity events: The effects of light deprivation on the seagrasses halodule pinifolia and halophila ovalis. Aquatic Botany. 65(1-4):105-121
- Greening H, Janicki A. 2006. Toward reversal of eutrophic conditions in a subtropical estuary: Water quality and seagrass response to N loading reductions in tampa Bay, florida, USA. Environmental Management. 38(2):163-178
- Larsson M, Ajani P, Rubio A, Guise K, McPherson R, Brett S, Davies K, Doblin M. 2017. Long-term perspective on the relationship between phytoplankton and nutrient concentrations in a southeastern australian estuary. Marine Pollution Bulletin. 114(1):227-238
- Gibbes B, Grinham A, Neil D, Olds A, Maxwell P, Connolly R, Weber T, Udy N, Udy J. 2014. Moreton Bay and its estuaries: A sub-tropical system under pressure from rapid population growth. In: Wolanski E. (Ed.) Estuaries of australia in 2050 and beyond. Springer. p. 203-222
- MacIntyre HL, Geider RJ, Miller DC. 1996. Microphytobenthos: The ecological role of the ”secret garden” of unvegetated, shallow-water marine habitats .1. Distribution, abundance and primary production. Estuaries. 19(2A):186-201. <Go to ISI>://A1996UX17600003
- Cahoon LB. 1999. The role of benthic microalgae in neritic ecosystems. Oceanography and marine Biology: an Annual Review. 37:47-86
- Grinham A, Gale D, Udy J. 2011. Impact of sediment type, light and nutrient availability on benthic diatom communities of a large estuarine Bay: Moreton Bay, australia. Journal of paleolimnology. 46(4):511-523
- Boynton W, Ceballos M, Bailey E, Hodgkins C, Humphrey J, Testa J. 2018. Oxygen and nutrient exchanges at the sediment-water interface: A global synthesis and critique of estuarine and coastal data. Estuaries and Coasts. 41(2):301-333
- Cowan JL, Boynton WR. 1996. Sediment-water oxygen and nutrient exchanges along the longitudinal axis of chesapeake Bay: Seasonal patterns, controlling factors and ecological significance. Estuaries. 19(3):562-580
- Eyre BD, Ferguson AJ, Webb A, Maher D, Oakes JM. 2011. Denitrification, n-fixation and N and phosphorus fluxes in different benthic habitats and their contribution to the N and phosphorus budgets of a shallow oligotrophic sub-tropical coastal system (southern moreton Bay, australia). Biogeochemistry. 102(1-3):111-133
- O’Neil J, Albert S, Osborne N, Shaw G, Heil C, Mulholland M, Bronk D. N acquisition by the toxic marine cyanobacterium lyngbya majuscula from moreton Bay australia and tampa Bay florida. International Society for the Study of Harmful Algae; 2004; Cape Town.
- Watkinson A, O’Neil J, Dennison W. 2005. Ecophysiology of the marine cyanobacterium, lyngbya majuscula (oscillatoriaceae) in moreton Bay, australia. Harmful algae. 4(4):697-715
- Ahern KS, Ahern CR, Savige GM, Udy JW. 2007. Mapping the distribution, biomass and tissue nutrient levels of a marine benthic cyanobacteria bloom (lyngbya majuscula). Marine and Freshwater Research. 58(10):883-904
- Cadée GC, Hegeman J. 1974. Primary production of the benthic microflora living on tidal flats in the dutch wadden sea. Netherlands Journal of Sea Research. 8(2-3):260-291
- Hewson I, O¹Neil JM, Heil CA, Bratbak G, Dennison WC. 2001. Effects of concentrated viral communities on photosynthesis and community composition of co-occurring benthic microalgae and phytoplankton. Aquatic Microbial Ecology. 25(1):1-10
- Round FE, Crawford RM, Mann DG. 1990. The diatoms. Cambridge University Press, Cambridge
- Sundbäck K, Snoeijs P. 1991. Effects of nutrient enrichment on microalgal community composition in a coastal shallow-water sediment system: An experimental study. Botanica Marina. 34(4):341-358
- Underwood GJ. 2002. Adaptations of tropical marine microphytobenthic assemblages along a gradient of light and nutrient availability in suva lagoon, fiji. European Journal of Phycology. 37(3):449-462
- Lockington JR, Albert S, Fisher PL, Gibbes BR, Maxwell PS, Grinham AR. 2017. Dramatic increase in mud distribution across a large sub-tropical emBayment, moreton Bay, australia. Marine pollution bulletin. 116(1):491-497
- Grinham A. 2006. Downstream effects of land use on shallow-water benthic microalgal communities in moreton Bay, australia and marovo lagoon, solomon islands University of Queensland. Brisbane
- Meyer-Reil L-A, Köster M. 2000. Eutrophication of marine waters: Effects on benthic microbial communities. Marine Pollution Bulletin. 41(1):255-263
- Armitage AR, Fong P. 2004. Upward cascading effects of nutrients: Shifts in a benthic microalgal community and a negative herbivore response. Oecologia. 139(4):560-567
- Defew E, Perkins R, Paterson D. 2004. The influence of light and temperature interactions on a natural estuarine microphytobenthic assemblage. Biofilms. 1(1):21-30
- Coates-Marnane J, Pausina S, Burton J, D. H, F. O, Olley J. in review,. Evidence for a regime shift in coastal diatom communities in response to anthropogenic nutrient loading. Estuaries and Coasts. Coates-Marnane, J., Pausina, S., Burton, J., Haynes, D., Oudyn, F., Olley, J. Coastal diatom community response to catchment land-use changes in Moreton Bay, east coast Australia. Marine and Freshwater Research, in review.
- Coates-Marnane J, Pausina S, Burton J, Haynes D, Oudyn F, Olley J. in review,. Coastal diatom community response to catchment land-use changes in moreton Bay, east coast australia. Marine and Freshwater Research.
- Coates-Marnane J, Olley J, Burton J, Sharma A. 2016. Catchment clearing accelerates the infilling of a shallow subtropical Bay in east coast australia. Estuarine, Coastal and Shelf Science. 174:27-40
- Albert S, O’Neil JM, Udy JW, Ahern KS, O’Sullivan CM, Dennison WC. 2005. Blooms of the cyanobacterium lyngbya majuscula in coastal queensland, australia: Disparate sites, common factors. Marine Pollution Bulletin. 51(1):428-437
- Johnson S, Abal E, Ahern K, Hamilton G. 2014. From science to management: Using Bayesian networks to learn about lyngbya. Statistical Science. 29(1):36-41
- Kehoe M, O’Brien K, Grinham A, Rissik D, Ahern K, Maxwell P. 2012. Random forest algorithm yields accurate quantitative prediction models of benthic light at intertidal sites affected by toxic lyngbya majuscula blooms. Harmful Algae. 19:46-52
- Elmetri I. 2003. Some chemical and physical factors controlling the growth of lyngbya majuscula : Implications for management of eutrophication in moreton Bay, queensland. The University of Queensland. Brisbane
- Hamilton GS, Fielding F, Chiffings AW, Hart BT, Johnstone RW, Mengersen K. 2007. Investigating the use of a Bayesian network to model the risk of lyngbya majuscula bloom initiation in deception Bay, queensland, australia. Human and Ecological Risk Assessment. 13(6):1271-1287
- García R, Johnstone RW. 2006. Effects of lyngbya majuscula (cyanophycea) blooms on sediment nutrients and meiofaunal assemblages in seagrass beds in moreton Bay, australia. Marine and Freshwater Research. 57(2):155-165
- Estrella SM, Storey AW, Pearson G, Piersma T. 2011. Potential effects of lyngbya majuscula blooms on benthic invertebrate diversity and shorebird foraging ecology at roebuck Bay, western australia: Preliminary results. Journal of the Royal Society of Western Australia. 94(2):171-179
- Olley J, Burton J, Hermoso V, Smolders K, McMahon J, Thomson B, Watkinson A. 2015. Remnant riparian vegetation, sediment and nutrient loads, and river rehabilitation in subtropical australia. Hydrological Processes. 29(10):2290-2300
- Olley J, Wilkinson S, Caitcheon G, Read A. Protecting moreton Bay: How can we reduce sediment and nutrient loads by 50%. Procedings of the 9th International RiverSymposium; 2006; Brisbane, Australia. p. 19.
- Grinham A, Deering N, Fisher P, Gibbes B, Cossu R, Linde M, Albert S. 2018. Near-bed monitoring of suspended sediment during a major flood event highlights deficiencies in existing event-loading estimates. Water. 10(2):34. https://doi.org/10.3390/w10020034
- Department of Environment and Science. 2018. Monitoring and sampling manual: Environmental protection (water) policy 2009. Brisbane: Queensland So.