-
Previous paper
Freshwater wetlands of Moreton Bay <i>Quandamooka</i> and catchments: Biodiversity, ecology, threats and management
Angela H. Arthington1, Steve J. Mackay1, Mike Ronan2, Cassandra S. James3, Mark J. Kennard1 -
This paper
Zooplankton of Moreton Bay
Sarah Pausina1,2 Jack Greenwood3, Kylie Pitt4, David Rissik5,6, Wayne Rochester2, Jennifer Skerratt7, Julian Uribe-Palomino2 and Anthony J. Richardson2,8 -
Next paper
Coral and micro-benthic assemblages from reef habitats in Moreton Bay
John M. Pandolfi1, Matt Lybolt2, Brigitte Sommer3, Roshni Narayan4, Paola Rachello-Dolmen5
Zooplankton of Moreton Bay
Authors
Sarah Pausina1,2 Jack Greenwood3, Kylie Pitt4, David Rissik5,6, Wayne Rochester2, Jennifer Skerratt7, Julian Uribe-Palomino2 and Anthony J. Richardson2,8Author affiliations
- School of Biological Sciences, University of Queensland, St Lucia Qld, 4072, Australia
- CSIRO Oceans and Atmosphere, Queensland Bioscience Precinct, St Lucia Qld, 4067, Australia
- School of Biological Sciences, University of Queensland, St Lucia Qld, 4072, Australia. (Retired Fellow)
- School of Environment and Science and Australian Rivers Institute, Griffith University, Gold Coast Qld, 4222, Australia
- BMT Eastern Australia Pty. Ltd, Brisbane, Qld 4000, Australia
- Australian Rivers Institute, Griffith University, Nathan, QLD 4111, Australia
- CSIRO Oceans and Atmosphere, CSIRO Marine Laboratories, Hobart Tas., 7001, Australia
- Centre for Applications in Natural Resource Mathematics (CARM), School of Mathematics and Physics, University of Queensland, St Lucia Qld, 4072, Australia
Corresponding author
s.pausina@uq.edu.auORCID
Sarah Pausina: https://orcid.org/0000-0001-7845-0296
Jack Greenwood: https://orcid.org/0000-0003-2882-7686
Kylie Pitt: https://orcid.org/0000-0002-2292-2052
David Rissik: https://orcid.org/0000-0002-1976-5324
Wayne Rochester: https://orcid.org/0000-0002-7315-9341
Jennifer Skerratt: https://orcid.org/0000-0002-7315-9341
Julian Uribe-Palomino: https://orcid.org/0000-0002-6867-2108
Anthony Richardson: https://orcid.org/0000-0002-9289-7366
Book
Zooplankton of Moreton Bay
Chapter
Research Paper Title
Zooplankton of Moreton Bay
Cite this paper as:
Pausina S, Greenwood J, Pitt K, Rissik D, Rochester W, Skerratt J, Uribe-Palomino J, Richardson A.. 2019. Zooplankton of Moreton Bay. In Tibbetts, I.R., Rothlisberg, P.C., Neil, D.T., Homburg, T.A., Brewer, D.T., & Arthington, A.H. (Editors). Moreton Bay Quandamooka & Catchment: Past, present, and future. The Moreton Bay Foundation. Brisbane, Australia. Available from: https://moretonbayfoundation.org/
DOI
10.6084/m9.figshare.8074364
ISBN
978-0-6486690-0-5
Abstract
Moreton Bay is a subtropical bay in south east Queensland that supports important populations of seabirds, marine mammals, reptiles and fish. Zooplankton, being small, are often overlooked, but are important nutrient cyclers and a critical link between primary producers and higher trophic levels. Here we synthesise available information on the zooplankton of Moreton Bay, from copepods to jellyfish, and describe their important roles in marine food webs. Zooplankton research in the Bay has a long history, focusing primarily on taxonomy, key taxa, seasonality, demersal zooplankton and jellyfish. Copepods dominate the fauna in the Bay, accounting for 74% of the permanent members. The temporary members of the zooplankton comprise early life stages of littoral species of molluscs, decapods, barnacles, annelids and fish. The dominant large zooplankton species is the jellyfish Catostylus mosaicus that swarms periodically, and its large biomass at times contributes significantly to nutrient cycling. Compared with immediately offshore, zooplankton in the Bay are more abundant but generally smaller in size and contain more meroplankton. In addition, the copepod community is more similar to communities of other tropical shallow coastal regions than zooplankton immediately offshore. Water quality models for the Bay have provided new insights into the variation of zooplankton in time and space that are difficult to investigate using standard sampling approaches. We conclude by highlighting key research gaps that need to be filled, namely the impact of flood events on zooplankton; the use of zooplankton as indicators of water quality to complement solely physico-chemical variables; harnessing historical data to assess the degree to which zooplankton communities have changed over recent decades; and the validation of the zooplankton components in water quality models.
Keywords: coastal ecology, subtropical, meroplankton, holoplankton, demersal, copepods, jellyfish, chaetognaths, larvaceans, IMOS
What is zooplankton?
The word ‘plankton’ is derived from the Greek planktos meaning ‘to drift’, and although most zooplankton are motile, none can progress against currents. Most plankton are microscopic, but some such as jellyfish may be up to 2m in bell diameter and can weigh up to 200 kg. Plankton communities are highly diverse, containing organisms from almost all kingdoms and phyla (Fig. 1). Plankton can be separated into the photosynthetic component (phytoplankton) and the animals (zooplankton). The permanent members of the zooplankton are known as holoplankton, and these include copepods (Subclass Copepoda), chaetognaths (Phylum Chaetognatha) and cladocerans (Superorder Cladocera). However, as almost all marine species shed their sperm and/or eggs directly into seawater to enhance dispersal, most marine animals are planktonic at some stage in their lifecycle. These temporary members of the zooplankton are called meroplankton. Like a caterpillar metamorphosing into a butterfly, many meroplankton look nothing like the adult form. Meroplankton, including mussel, barnacle and fish larvae, are more common in coastal areas where their progenitors live, and are generally more abundant at night than during the day because many species spawn then and others move up in the water column during the night.
Figure 1. Images of mixed zooplankton from Moreton Bay. Images: Julian Uribe-Palomino. (Click images to enlarge).
Why is zooplankton important?
Zooplankton are critical to the functioning of estuarine and coastal food webs because of their sheer abundance and vital ecosystem roles. The most prominent zooplankton, the copepods, could be the most abundant multicellular animals on Earth, perhaps 1,000 times more abundant than insects (1). The high phylogenetic diversity of zooplankton contributes to their diverse ecosystem functions. Arguably, the most important role of zooplankton is as the major grazer in food webs, providing the principal pathway for energy from primary producers (the phytoplankton) to larger consumers such as fish, marine mammals, jellyfish and sea turtles. Interestingly, some of the largest animals in the ocean, such as baleen whales, feed solely on zooplankton. This is in stark contrast with terrestrial ecosystems, where the largest animals are generally herbivores.
Zooplankton not only support the large, highly visible, and charismatic components of ocean food webs, but also the microbial community. Their feeding mechanisms, secretions and excretions play an important role in nutrient cycling (2, 3) and support microbial (4, 5) and phytoplankton production. Microbes colonise zooplankton faecal pellets and carcasses (3), making them rich sources of organic carbon for detrital feeders in the water column and benthos. In shallow coastal regions, demersal zooplankton play an important role in transferring energy and nutrients between the sea bottom and water column (6), and are also food for fish whilst in the water column (7, 8). Zooplankton grazing has the potential to exert selective pressure on phytoplankton community dynamics (9–13) that may determine the fate of algal blooms. Understanding the relationship between environmental drivers and zooplankton dynamics is an important step in safeguarding the ecological and economic resources of coastal regions and their resilience to anthropogenic nutrient enrichment and climate change.
History of zooplankton research in Moreton Bay
Although there has been relatively little zooplankton research in Australia over the past century compared with temperate regions in the northern hemisphere (for an example see (14)), Moreton Bay has had a surprising number of studies. The first studies were limited in area and descriptive. The earliest account of zooplankton research in Moreton Bay is from a January 1938 expedition by Laing (15), who undertook six net hauls east of Peel Island. She briefly described the plankton composition in broad taxonomic groups and noted a day/night difference. This was followed by Munro (16), who studied the general composition of zooplankton in Waterloo Bay and the night-time emergence.
Zooplankton studies published between the 1960s and the late 1990s have been reviewed by Greenwood (17). Succeeding zooplankton work by Greenwood in 1999 (18) formed a component of the broader Moreton Bay Study initiated by stakeholders to inform improvement of water quality for the Bay and its estuaries. While the taxonomic resolution was low, it extended the knowledge base for zooplankton abundance, size fractions and night/day population differences upstream to the Bremer River, northward to Deception Bay and southward to Pelican Banks.
There has been relatively little work in Moreton Bay in the 21st century. Greenwood (19) investigated the demersal zooplankton of the Brisbane River estuary and found that dredging led to a significant decline in zooplankton abundance, but had little impact on composition or distribution. In a study of the bacterioplankton using molecular techniques, Hewson and Fuhrman (20) found that some bacterioplankton taxa were restricted to distinct environments, whereas others had a ubiquitous distribution from the Brisbane River to the outer Bay. Recently, Uribe-Palomino et al. (21) described a new, tiny (2 mm) jellyfish species of the genus Merlicertissa whose holotype was collected in the Bay.
Dominant taxa
Copepods
As in most subtropical water bodies (22), copepods numerically dominate the fauna in the Bay, accounting for 74% of the holoplankton and 50% of the total zooplankton (23). The copepod community is always dominated by calanoids (average 76% of total copepods). Approximately half of the 68 calanoid species recorded by Greenwood (24) were found in fewer than 5% of the samples, with just 11 species present in 50% or more of the samples. These 11 species were also numerically important components of the zooplankton. Greenwood (24) found that the most frequently captured calanoid copepods in Moreton Bay, listed in descending order were, Pseudodiaptomus mertoni, Tortanus barbatus, Temora turbinata, Acartia pacifica, Bestiolina similis, Acartia tranteri, Parvocalanus crassirostris, Acrocalanus gibber, Centropages furcatus, Pseudodiaptomus colefaxi, and Calanopia australica.
Interestingly, two species in each of two genera were in the top 11 species, raising the question of how they coexist despite competing for shared resources. For example, the dominant calanoid copepod species, A. tranteri, an estuarine species endemic to Australia, and the more cosmopolitan, estuarine-coastal A. pacifica, are from the same genus. It is thought they are able to coexist because of seasonal and spatial niche separation (25). A. tranteri was typically dominant in colder temperature waters (14–21°C) and lower salinities between 30–36.5‰, whereas A. pacifica was dominant above 22°C and from 34–36.5‰. The same study similarly concluded P. colefaxi was more of a generalist, with broader temperature and salinity preferences, than its specialist congenitor P. mertoni (25).
Greenwood (23) found cyclopoid copepods typically constituted one-fifth of the total copepod fauna when collected using a 195µm mesh net. However, these characteristically smaller copepod species were sometimes dominant during the Task Plankton Trophodynamics Study (18), which used finer mesh nets. Thwin’s (26) time series work recorded 34 species of cyclopoids, with 10 of these in <2% of the samples. The three small species Oithona brevicornis, Oithona nana and Coryceaus andrewsi were the most frequent constituents of the cyclopoid fauna.
Globally, a mere 0.5% of described harpacticoid species permanently inhabit the pelagic realm (27). Unsurprisingly, harpacticoids constitute the smallest fraction of the copepod fauna in Moreton Bay, averaging just 2.7% and 1.4% of the copepod and total zooplankton abundances, respectively (23). Neritic and oceanic species such as Euterpina acutifrons, Microsetella spp., Macrosetella gracilis, Goniopsyllus rostrata (originally identified as Clytemnestra rostrata in (28)) and Metis holothuriae (probably benthic and associated with seagrass) have been collected in the Bay. Interestingly, M. gracilis uses the colonial cyanobacterium Trichodesmium both as a food source (29) and buoyant substrate for juvenile development (30). Trichodesmium is a major contributor to primary productivity in oligotrophic tropical and subtropical oceans (31), and periodically forms dense surface slicks in the Bay (32). M. gracilis may thus be an important trophic link between Trichodesmium and higher trophic levels in the more oligotrophic areas of the Bay, such as towards the eastern barrier islands.
Other zooplankton
Greenwood (23) found that the most numerous holoplanktonic groups, in order of numerical importance, were the appendicularians, cladocerans, chaetognaths and cnidarians. Appendicularians (larvaceans, (tunicates)) occasionally represented 60% of the non-copepod zooplankton. The Moreton Bay population was dominated by Oikopluera longicauda, with Oikopluera doica also common and Fritillaria pellucida rare (23). While often outnumbered by copepods, growth rates for larvaceans typically surpass those of copepods at the same temperature (33, 34). Larvaceans are one of the few metazoans to efficiently feed on the picoplankton (0.2–2 µm-sized particles), which are filtered from the water column using a mucous feeding structure known as a ‘house’ (35). Their filtration rates are high, and once they are clogged with phytoplankton, bacteria, ciliates, detritus and faecal pellets, houses are shed and replaced. New houses are secreted regularly; Sato et al. (36) observed house renewal rates from 2–40 per day for ten species from Tokyo Bay, Japan. O. longicauda produces and discards up to 24 per day and the carbon content of particle-loaded, discarded houses corresponds to 18% of somatic carbon (36). Given their abundances and ubiquity, larvaceans are thus an important source of secondary production in the coastal and oceanic systems they inhabit. In addition, larvacean houses are colonised by bacteria and flocculate with other organic detritus, forming marine snow (37). The subsequent breakdown of discarded houses not only makes organic matter available for remineralisation by the microbial community (37), but these macroscopic aggregates provide food for copepods (38) otherwise unable to consume picoplankton-sized particles due to the limitations of their feeding structures (39). Discarded houses thus represent an important link between the microbial and classical food webs.
To date, four of the world’s eight known marine cladocera species have been recorded in Moreton Bay (23, 28). Cladocerans are important links in the microbial loop because they are capable of feeding on organisms ranging from <2 µm (flagellates) up to 100 µm (diatoms, dinoflagellates and ciliates) (40–42) and in turn are preyed on by higher trophic consumers such as chaetognaths (43) and planktivorous fish (44) in classical food webs. Owing to their short developmental times and unique ability among the planktonic crustaceans to reproduce asexually (by parthenogenesis), cladoceran populations can respond rapidly to favourable environmental conditions and reach high densities (42). Moreton Bay’s cladoceran population is typical of other neritic subtropical waters, with the dominance and almost constant presence of Penilia avirostrus, one of the more abundant and geographically widespread species (45). Highest observed densities were 635 m-3 but annual average was one-tenth of that maximum at 64 m-3 (23). As a group, the cladocerans averaged 3.8% of the total zooplankton (23). Other species captured in the Bay were Pseudevadne tergestina, Evadne nordmanni and Pleopis polyphemoides.
Chaetognaths are known as arrow worms and are raptorial carnivores. They are found across most marine habitats including estuaries, bays, and open oceans from polar to equatorial waters (46). They are ambush predators, and prey include copepods and cladocerans (47). Globally they are often reported in abundances second only to copepods in the mesozooplankton (48, 49), and indeed they are among the dominant taxa in Moreton Bay. This group formed 3.4% and 5% of the total zooplankton and holoplankton, respectively, in a study in years 1963–1966, with notable species being the cosmopolitan Flaccisagitta enflata and Indo-Pacific-distributed Aidanosagitta neglecta (23).
The meroplankton in Moreton Bay are largely composed of early life stages of littoral species. In Greenwood’s (23) collections, the most numerous meroplankton were the larvae of molluscs, decapods, barnacles and annelids. Only the copepods outnumbered molluscan veliger larvae, whose abundances averaged 23% of the total zooplankton. Gastropod veligers, found throughout the year, were more numerous than bivalve veligers. The decapod component was diverse and dominated by brachyuran zoeae, but included penaeid larvae and Lucifer and porcellanid crab zoeae. Barnacle (cirripede) nauplii and cyprids averaged 38 individuals m–3 and were present year-round. However, nauplii were much more common than cyprids, which are a more mature, briefer and non-feeding developmental stage. Many of the planktonic annelids, which were present in all months were spionid and polynoid polychaete larvae. Other, less common, polychaetes included the Terebellidae and the holoplanktonic Tomopteris sp.
Cnidarians are a diverse and widespread group of largely colonial invertebrates that includes many captivating forms such as corals, sea fans, sea anemones and the floating Portuguese man o’war, (aka blue bottle). All cnidarians possess nematocysts, the specialised stinging organelles feared by bathers. Some cnidarians have only a sessile polyp stage (e.g. corals), others have only a pelagic medusa stage (e.g. some true jellyfish species), but many cnidarians have alternation of generations, switching between the asexual polyp and the sexual pelagic medusa stage. Gelatinous, planktonic cnidarians include the siphonophores (Class: Hydrozoa), box jellyfish (Class: Cubozoa) and true jellyfish (Class: Scyphozoa).
The cnidarian community from Moreton Bay has been characterised in seven studies, a number of which are unpublished theses or reports, and most are taxonomic accounts (16, 23, 50-54). Gelatinous animals are notoriously difficult to sample effectively; they are often too large for most sampling gear, or so small and delicate that they disintegrate upon capture, and their mucus has a tendency to clog net mesh and compromise entire samples.
About 58 species of cnidarians have been recorded in Moreton Bay, 52 of which are reported and reviewed by Gershwin et al. (54) from collections and literature, including descriptions for seven new species and new distribution records. It is remarkable that such an extensive appraisal of Cnidaria exists for the Bay, although this taxon list does not include the species Diphyes subtiloides and genera Corymorpha sp. (as Steenstrupia sp.), Sarsia sp., Melicertissa sp. (as Melicertiasa sp.), Mitrocomidae sp. (as Cosmetira sp.), Lensia sp. found by Munro (16). Gorman (52) sampled cnidarians and ctenophores from seven stations in the Bay and, using a wider net than Greenwood (23), described a more abundant and more diverse community dominated by hydrozoans, with many fewer scyphozoans. The hydrozoans Octophialucium medium and Aequorea australis were most abundant by one to two orders of magnitude (52). Interestingly, neither of these species was recorded in Payne’s (50) five common species for the Bay, highlighting the patchiness in space and time of the planktonic cnidarians.
Siphonophores and other hydrozoans have been found year-round within the Bay and average combined densities have reached 43m–3 (23). By contrast, the larger Catostylus and Cyanea scyphozoans are observed sporadically, with Catostylus occasionally forming dense swarms (23, 52). Thus, while they represent only a minority of the planktonic fauna by number, cnidarians can attain significant biomass in the Bay. Other notable taxa include the hydrozoans Physalia physalis (bluebottle) and Velella velella (by-the-wind-sailor), both cosmopolitan, buoyant, bright blue, and conspicuous when blown onto beaches. There is currently little known about the micro-medusae (mature form < ~5 mm bell diameter) from this region.
Seasonality
Moreton Bay is a subtropical embayment and thus is likely to have a dampened seasonal cycle compared with higher latitudes. Thwin (26), for example, observed that cyclopoid copepods formed three main assemblages: (i) estuarine species, (ii) inshore species present at all times, and (iii) seasonal offshore intruders. In other words, the primary division of these groups was based on estuarine and non-estuarine species, with temperature only secondarily driving a division into cold and warm water groups.
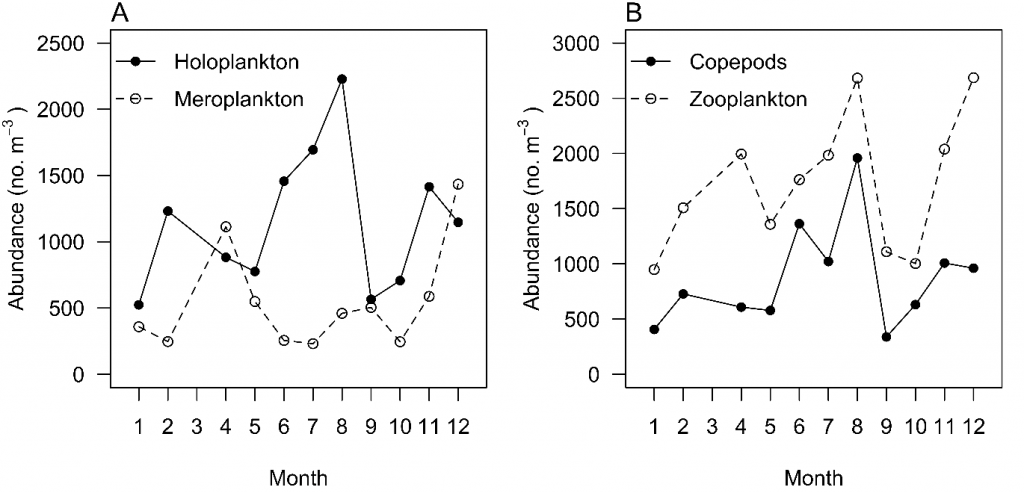
Greenwood (23) found that total zooplankton, total copepods, holoplankton, and meroplankton did not show typical seasonal patterns, with all having multiple peaks throughout the year (Fig. 2). Many taxa, however, exhibit strong seasonal patterns. Meroplankton such as bivalve, gastropod, decapod, polychaete and echinoderm larvae contributed most to total zooplankton abundances in summer (23), and Tafe (55) found cumacean species richness was lowest in winter and highest in summer. There is strong evidence for seasonality in copepod species richness, with more oceanic copepods during late summer to winter, which leads to a peak of copepod species richness in winter (24). This is probably due to the annual intrusion of oceanic water that delivers oceanic species and increases species richness in Moreton Bay (24) (Fig. 3). Other oceanic species of chaetognaths, larvaceans, and salps have been recorded in the Bay during this period; for example, the chaetognath Flaccisagitta enflata exhibits a positive correlation with salinity, suggesting oceanic water intrusion (23).

Peak abundances of the cladoceran Penilia avirostrus were found in cooler months (23), in contrast to other subtropical bays where the species typically peaks in warmer months (55–57). We have no indication of what drives this difference, although Penilia populations in tropical Kingston Harbour, Jamaica, are not generally food limited (45). Rose et al. (45) could find no seasonal patterns and no correlation of abundances with chlorophyll concentrations, nor indeed with any measured physical variables within the Harbour. They surmised this neritic population may instead be regulated by predation, which may likewise be the case in Moreton Bay.
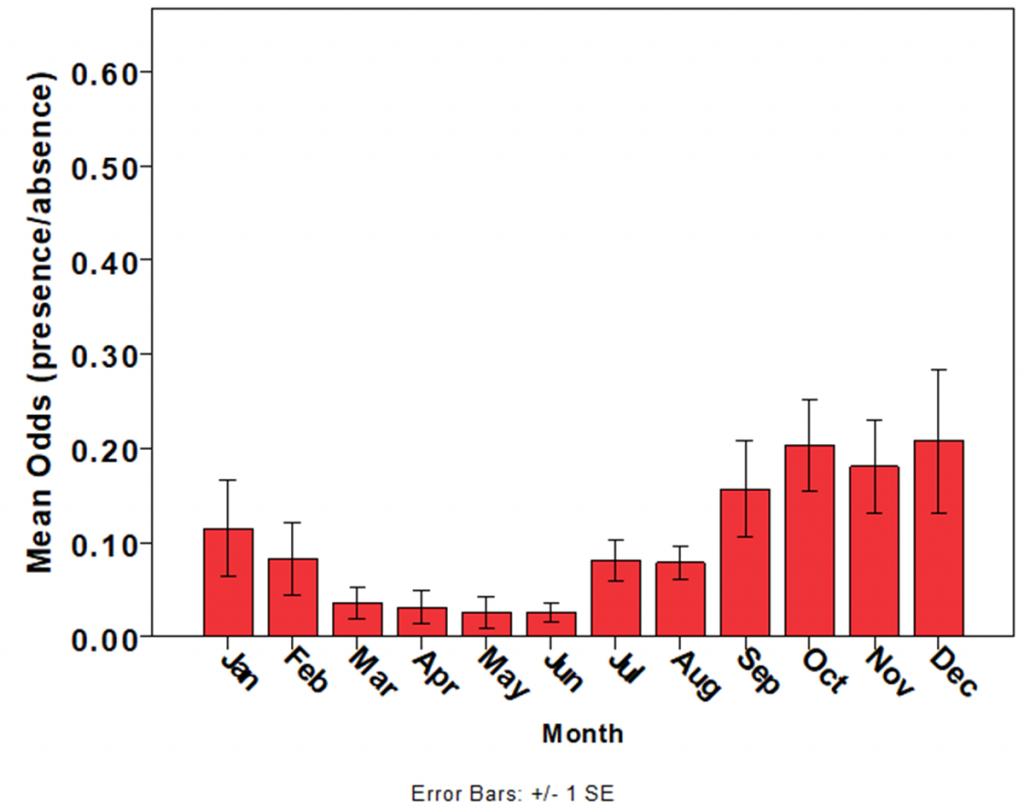
The most robust dataset on jellyfish abundance in Moreton Bay has been collected by the Queensland Government’s Ecosystem Health Monitoring Program, which has recorded the presence or absence of C. mosaicus each month since 2002 at >100 sites in Moreton Bay. Within years, C. mosaicus exhibits strong seasonality, with juvenile medusae recruiting during spring and summer and populations declining in autumn, although medusae may occasionally be encountered during winter (Fig. 4) (56). Limited data exist for other species of jellyfish in Moreton Bay.
Despite limited evidence of strong repeated seasonal cycles, most studies have only been conducted over a single year. Further studies are needed to confirm the degree of seasonality, and whether multiple peaks are indeed consistent each year or simply indicative of a subtropical environment with periodic and ephemeral productivity pulses. Subtropical estuaries often have more pronounced spatial variation in community structure of subtropical zooplankton across salinity and water quality gradients than seasonal differences (57). Many studies have grouped taxa together, which can hide seasonality if there are different peaks and troughs in various taxa, so species-level analyses might prove more informative. No work has investigated the association between the summer rainy season and the elevated freshwater inputs and nutrient loads at that time.
Demersal zooplankton
Estuaries, bays and coastal waters the world over contain resident zooplankton that dwell in, on or just above the seafloor during the day and emerge into the water column at night. This demersal fauna has received considerable attention in Moreton Bay, and numerous studies have shown pronounced diel changes in the composition of its zooplankton. Jacoby and Greenwood (58) used emergence traps, re-entry traps and surface tows to study vertical migration patterns in the zooplankton. While they found differences in taxa and abundances captured using different nets and across seasons, they observed a general pattern of emergence at night using surface tows (Fig. 5). A total of 17 of the 23 taxa investigated showed night-time emergence, with the larger zooplankton taxa, especially crustaceans, exhibiting nocturnal emergence (58). Mysids, a shrimp-like crustacean, showed the strongest emergent signal at night. Laing (15) and Munro (16) similarly collected mysids more frequently in night-time hauls and in higher abundances than those taken in daylight. Copepods of the genus Pseudodiaptomus are well known to be demersal (59, 60), with varying degrees of affinity to the substratum (58). Pseudodiaptomus colefaxi is a species of calanoid copepod dominant in the Bay whose abundances are higher in surface waters at night. Its congener, P. mertoni, was found throughout the water column during the day but in greater numbers at night. More mature developmental stages of Pseudodiaptomus spp. had demersal habits similar to those of adults. Emergence was observed in other copepods including harpacticoids, Oithona and Acartia spp., as well as mollusc larvae (e.g. bivalve and gastropod veligers).

Jacoby and Greenwood (58) found substratum type to be an important factor governing emergence patterns of demersal plankton. Moreton Bay has diverse subtidal habitats including coral, seagrass beds, and sandy, muddy and rocky bottoms. Substratum type determined how many and which taxa emerged, with 41 of the 43 demersal taxa studied emerging in greater numbers from more structurally complex coral and seagrass than from the more uniform coral rubble and mud habitats. Further, for most taxa, they recorded emergence in densities about 10 times greater than for taxa from their Heron Island lagoon study (61). This has important ramifications for zooplankton productivity and links to higher trophic levels, particularly given recent studies documenting increases in terrestrially derived mud content and mud distribution across Moreton Bay (62, 63).
Emergence of demersal zooplankton is also common in the lower reaches of rivers entering Moreton Bay. Larger zooplankton such as the sergestid shrimp Acetes sibogae, present throughout the Bay but primarily concentrated in the lower reaches of its tributaries, spends daylight hours at or near the sediment–water interface and migrates into the water column at night on flood tides (64). Greenwood et al. 2002 (19) sampled the near-bottom zooplankton populations in the Brisbane River during a study on the impacts of gravel extraction, and 33 of 90 taxa captured during daytime tows were generally considered to be demersal. Common demersal zooplankton included the copepods Gladioferens pectinatus, Pseudodiaptomus spp., Stephos morii and Brianola, and isopods, amphipods, mysids, tanaids, numerous decapods, and planktonic stages of fish.
There are numerous drivers of emergence in demersal zooplankton. Many demersal zooplankton emerge in the water column at night to feed on phytoplankton, holoplankton or other emergent zooplankton. During the day, demersal zooplankton tend to return to the substrate to hide from visual predators. Nocturnal migrators tend to be relatively large, with highly discernible swimming movements, rendering them vulnerable to predation by visual predators such as planktivorous fish (7, 65). Emergence from the seafloor can also have the benefit of population maintenance in a region. For example, the sergestid shrimp Acetes sibogae times its emergence at night to the flood tide to maintain its position in the estuary (64). Some benthic crustaceans emerge at the same time to increase the likelihood of finding a mate (66). Smaller zooplankton or transparent larval forms are less subject to visual predation and often show weaker emergence or vertical migration. Emergence into the water column also has the benefit of facilitating passive transport to exploit new feeding regions.
Regardless of its cues and adaptive significance, this cyclic movement of living organisms is an important process in bentho-pelagic coupling as it transfers energy from surface waters to the substrate and vice versa. Larger crustaceans such as mysids and sergestids, for example, are important dietary components of resident juvenile fishes (65). Understanding diel patterns in the contribution of such taxa to the planktonic population is necessary for establishing their role in trophic pathways.
Jellyfish ecology
Jellyfish are common and conspicuous members of Moreton Bay zooplankton assemblages. Scyphozoan jellyfish are the most visible and some species form spectacular blooms that comprise a substantial proportion of the pelagic biomass. Jellyfish are voracious predators of other zooplankton, including other jellyfish species (67) and act as hosts for numerous species of fish and invertebrates (67, 68) and so have an important role in the ecology of Moreton Bay. They are also likely to influence nutrient dynamics within the Bay, particularly when large blooms of jellyfish collapse suddenly and decompose on the sea floor. Moreover, due to their sheer numbers, they can sometimes impart substantial socio-economic effects. For example, in 2005, the aircraft carrier USS Ronald Reagan departed the Port of Brisbane earlier than scheduled when the blue blubber (Catostylus mosaicus), Moreton Bay’s most abundant large jellyfish, interfered with the cooling intakes of the ship.
Inter-annual variation
Populations of jellyfish typically exhibit strong inter- and intra-annual variability in abundance. Data from the Queensland Government’s Ecosystem Health Monitoring Program hints that populations of C. mosaicus, like many other species of jellyfish (69), exhibit distinct cycles of abundance, with jellyfish being very abundant for several years, followed by years when jellyfish are scarce or absent (Fig. 6) (56). Until data tracking multiple complete cycles are available, cyclic behaviour of C. mosaicus populations in Moreton Bay cannot be confirmed.

Trophic interactions
Jellyfish are voracious predators of zooplankton and, when abundant, can influence the dynamics of zooplankton and phytoplankton communities (70, 71). As jellyfish swim continuously, they capture prey throughout the day and night and thus also consume nocturnal emergent taxa (67). In Moreton Bay, C. mosaicus captures about 50% of the zooplankton taxa that are present in the water column (67). Gastropod and bivalve veligers, copepods, brachyuran crab zoeae and amphipods are the most common species captured by C. mosaicus in the Bay, whilst ostracods and barnacle nauplii either evade capture or are rejected by the jellyfish (67).
Until recently, jellyfish were considered to be trophic ‘dead ends’ as they were thought to be consumed by just a few species that specialise on feeding on them (such as leatherback turtles and sunfish). The diversity of animals that prey on jellyfish, however, is likely to have been grossly underestimated since jellyfish tissue decomposes rapidly in the guts of predators and is difficult to identify using traditional gut content techniques. Emerging technologies, such as using cnidarian-specific mtDNA assays (72) is revealing that a much greater diversity of animals prey upon jellyfish than once thought and this is also likely to be the case in Moreton Bay. Having healthy populations of jellyfish may thus be important in sustaining populations of a diverse range of species, including the numerous species of sea turtle that inhabit Moreton Bay.
Commensal relationships
A diversity of organisms associate with jellyfish in Moreton Bay, including dinoflagellates (zooxanthellae; 73), fish (68), copepods (74), and anemones and isopods (75). The relationship between jellyfish and the animals they host varies and includes parasitism, whereby the associates may feed on or deposit their eggs and larvae within the tissues of the host jellyfish, thus harming it (e.g. Brown et al. (75)); commensalism, whereby one partner may benefit from the association but the other partner is unharmed (such as the fish Trachurus novaezelandiae that appears to shelter under the umbrella of C. mosaicus but not harm it (76)); and symbiosis sensu stricto, in which both partners benefit (such as the dinoflagellates that associate with Cassiopea sp.). Jellyfish, therefore, are likely to have a major role in supporting pelagic biodiversity within Moreton Bay. Indeed, the diversity of organisms that associate with jellyfish in the Bay is likely to be much greater than stated here, since in other regions of Australia, C. mosaicus associates with anemones and isopods (75), and intermediate stages of digenean (Class: Trematoda) parasites (77).
Influence in nutrient cycling
Due to their ‘boom and bust’ population dynamics, and their sheer abundances, jellyfish have a major role in biogeochemical cycling (78). As populations of jellyfish grow, they assimilate carbon and nutrients from their prey, and excrete nitrogenous wastes and dissolved organic carbon into the water column. Blooms of jellyfish thus represent significant repositories of carbon and nutrients that are subsequently released when the populations collapse during autumn. Senescent jellyfish rapidly sink to the sea floor and may be consumed by benthic scavengers such as fish, or be remineralised by bacteria (79). In Moreton Bay, jellyfish carcasses are more likely to be scavenged than remineralised, since potential scavengers are abundant. Winds and tidal currents often strand large numbers of jellyfish on the beaches of Moreton Bay (Fig. 7). These stranding events are likely to provide important, although episodic, trophic and nutrient subsidies to the sandy shore environments, where in situ levels of productivity are typically low.

Zooxanthellate jellyfish influence nutrient cycling differently than non-zooxanthellate species since the nitrogenous wastes and carbon dioxide excreted by the host jellyfish are almost entirely used by their symbionts and thus recycled internally within the holobiont (80). Moreover, zooxanthellate jellyfish can assimilate dissolved inorganic nitrogen and phosphorus from the water column (81).
How do zooplankton differ inside and outside Moreton Bay?
Zooplankton in coastal bays are often substantially different from neighbouring oceanic regions. The zooplankton community in Moreton Bay exhibit lower biomass, higher abundances and lower species richness than at an oceanic site outside the Bay according to data from the Integrated Marine Observing System (IMOS) National Reference Station off North Stradbroke Island (Fig. 8A–C). This is typical of zooplankton communities in coastal waters at lower latitudes, which generally exhibit higher abundances and lower species richness in more eutrophic, inshore waters than in more oligotrophic offshore waters (57, 82, 83). Copepods in Moreton Bay are smaller than in oceanic waters (Fig. 8D), which explains the lower biomass yet higher abundance in Moreton Bay relative to the oceanic site; eutrophic bays commonly have smaller zooplankton (84, 85). Both the total abundance of meroplankton and the ratio of meroplankton to holoplankton are higher in the Bay than outside, as expected given more larvae of littoral forms (e.g. molluscs, barnacles, prawns) (Fig. 8E, F)

How does Moreton Bay compare with other areas in Australia?
The IMOS network of National Reference Stations (NRSs) provides an ideal dataset for comparing Moreton Bay zooplankton with the fauna of other areas around Australia. We analysed copepod abundance and species composition from samples from Peel Island in Moreton Bay, collected with a 100µm mesh net that was used throughout the IMOS NRS network, with data from various IMOS stations around Australia (12 samples from Peel Island in 2009 and 388 samples from the NRS sites 2010–16). The first two Principal Components Analysis (PCA) axes accounted for 14% and 7% of the variance in species composition, respectively (Fig. 9). There was strong differentiation in the copepod assemblages among stations. Copepod communities on the right-hand side of the PCA are from temperate regions

and those on the middle and left side are from subtropical and tropical regions. Interestingly, Moreton Bay (Peel Island) communities are distinct from those of neighbouring North Stradbroke Island, and are most similar to Darwin Harbour communities, followed by SS Yongala off Townsville. Based on an index of species indicator value (86) calculated for sites and combined pairs of sites, species characteristic of Peel Island and Darwin Harbour (as a pair) included the copepods Parvocalanus crassirostris, Oithona attenuata, Oithona simplex and Euterpina acutifrons. Species characteristic of Peel Island, Darwin Harbour and Yongala (as a group) were P. crassirostris and O. simplex. These three sites are situated close to land prone to extreme and rapid fluctuations in freshwater run-off, and hence tend to be inhabited by species adapted to variability in temperature and/or salinity. P. crassirostris, for example, is a well-known euryhaline and eurythermal marine copepod (57, 87–90). Species that set the two south east Queensland sites apart included P. crassirostris, Oithona brevicornis and Oithona australis at Peel Island versus Oncaea venusta, Clausocalanus furcatus and Oithona plumifera at North Stradbroke Island. The North Stradbroke Island IMOS station, being situated outside the Bay, is more likely to experience variability associated with coastal upwelling and oceanic intrusions than freshwater flows and thus the copepods tend to be from mixed coastal and oceanic communities. C. furcatus, for example, is typically associated with warm, nutrient-poor oceanic waters (91).
Copepod species diversity in Moreton Bay is very high compared with Australian tropical and temperate bays such as Darwin Harbour and Port Phillip Bay respectively, while mean abundances in Darwin Harbour substantially outnumber those in more southern sites (57, 88, 89, 92).
Modelling zooplankton
The South East Queensland Regional Water Quality Management Strategy was prepared by the Healthy Waterways Partnership in 2001 to address concerns about declining water quality. The Strategy identified a need for a model to determine management and remediation strategies for water quality in the Bay system. A Receiving Water Quality Model (RWQM) (93) related the transport and fate, including uptake in plankton, of nutrient sources (nitrogen and phosphorus) in the water and sediment. This model is used as a predictive tool to assess environmental and economic impacts of different management scenarios. The RWQM has two size-based groups of zooplankton. The first is ‘small zooplankton’, which represents microzooplankton <200 μm in size such as heterotrophic flagellates, tintinnids, ciliates, rotifers, small copepod nauplii and polychaete larvae. They are mobile, feed on small phytoplankton and have rapid turnover rates. The other group is ‘large zooplankton’, which represents mesozooplankton such as copepods and small fish larvae. They are mobile, and feed on large phytoplankton, microphytobenthos and dinoflagellates. ‘Large zooplankton’ have a slower growth rate than small zooplankton, which results in a lag between enhanced primary and secondary production. For both zooplankton groups, grazing success depends on the food encounter rate, which in turn is related to zooplankton swimming speed, food size and density. Excretion and inefficient feeding return dissolved and particulate material to the water column. Zooplankton mortality and predation by higher consumers, such as fish, are not simulated within a nutrient-phytoplankton-zooplankton-detritus model structure and so are represented using a closure term.
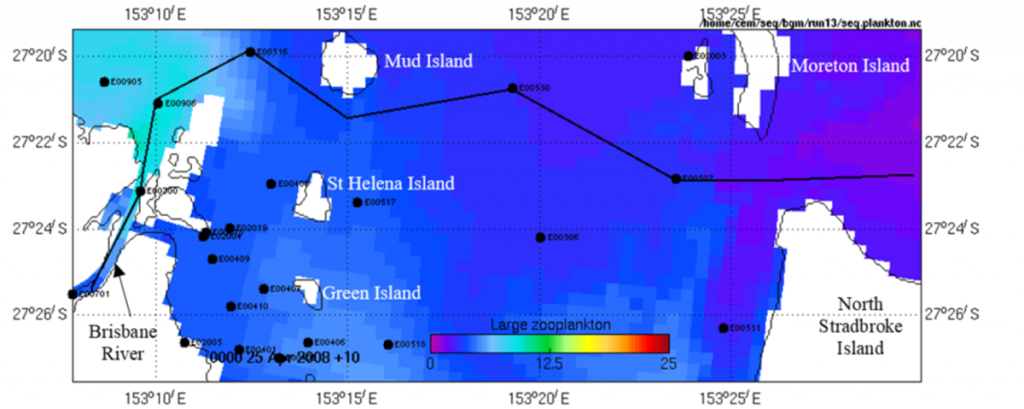
Although the RWQM has had little zooplankton validation to date, it provides insight into the temporal and spatial variation of zooplankton that has not previously been available. There were several notable features (Figs. 10, 11). There is higher biomass of ‘large zooplankton’ than ‘small zooplankton’. There is also higher biomass of both ‘large zooplankton’ and ‘small zooplankton’ in the western compared with the eastern Bay, a consequence of the east–west gradient in chlorophyll levels from eutrophic conditions in the west to oligotrophic conditions in the east. Generally, there is higher zooplankton biomass in spring and summer than in autumn and winter, as wet season flows during spring/summer are important sources of nutrients that stimulate algal blooms. Finally, the seasonal and spatial changes are more marked in the ‘large zooplankton’ than in the ‘small zooplankton’. Many of the model’s findings remain to be validated through fieldwork, but it has provided insight into spatial and temporal variation of the zooplankton community overall.

Key recommendations for research priorities
While the primary focus of zooplankton research in Moreton Bay has been documenting the fauna (e.g. 26, 94-98), and in some cases recording species new to science (e.g. 99), earlier studies have examined the compositional change in zooplankton across the estuarine axis of Moreton Bay (23, 24, 100), and the contribution of emergent demersal forms to the plankton (58). However, there remain several key knowledge gaps that need to be filled. First, there has been no work investigating the seasonality of zooplankton associated with the wet season in south east Queensland (November to April) that elevates freshwater inputs and nutrient loads. The RWQM suggests that there should be higher zooplankton biomass in spring/summer associated with increased rainfall, but this has not been tested in the field as almost all studies have been shorter than a year and none have traced the evolution of zooplankton biomass and community following a flood event.
Second, small zooplankton needs to be sampled more thoroughly. The RWQM suggested that small zooplankton are abundant and cosmopolitan in the Bay, yet historical zooplankton studies have used a relatively large mesh size of 195 µm and have thus under-sampled smaller taxa. Smaller copepods are likely to dominate when finer mesh samples are collected, and these species are likely to exert high grazing pressure on the abundance and biomass of phytoplankton assemblages than previously thought. In addition, copepods feed not only on the classical food chain (i.e. phytoplankton) but also on heterotrophic protists. Estimates of zooplankton biomass and abundance will be much higher once the smaller zooplankton component is adequately sampled.
Third, physico-chemical status and phytoplankton biomass are used as ecosystem indicators in the current Ecosystem Health Monitoring Program, particularly in response to nutrients, but zooplankton have not been considered. Zooplankton could be ideal ecosystem indicators because they are ubiquitous and are responsive to environmental change, eutrophication, pollution and climate change. For example, changes in zooplankton community size structure have been linked with eutrophication (84, 101).
Increasing nutrient concentrations can also cause changes in zooplankton biomass (101), abundance (85), size spectra (84) and feeding behaviour (88). In response to nutrients, there have been pronounced shifts in copepod community structure from dominance of larger to smaller species: in mesocosms in Norway (102); in Kingston Harbour, Jamaica (87); in Chesapeake Bay, United States (85); and in Tokyo and Osaka bays in Japan (84). Indeed, Tokyo and Osaka bays experienced an increase in nutrient loading in the 40 years following World War II that resulted in the replacement of large copepods by small ones (84). The lowest median body weight of the zooplankton community occurred closest to shore within each bay and steadily increased with distance from inshore stations. Median weight for the zooplankton community in the comparatively less eutrophic Osaka Bay was one to two orders of magnitude higher than for Tokyo Bay. All these patterns are potential candidates for ecosystem indicators of nutrient loading focused on zooplankton. Mouillot et al. (103) argue that alternative descriptors such as body size, proportions and diversity of various functional groups, and productivity of key species, have greater local relevance than taxonomic-based approaches such as indicator species, taxon richness and diversity indices. These characteristics could be analysed to assess their potential to supplement more traditional abiotic indicators of ecological status.
Fourth, there is opportunity to assess how changes in environmental conditions, particularly nutrients over >50 years, could influence the zooplankton community. Most of the historic work of Jack Greenwood on zooplankton was based on samples collected in the 1960s and there are several publications with data tables. If the challenges associated with accounting for different sampling methods can be overcome, such comparative studies can provide unique insights into long-term ecosystem change in response to environmental change.
Last, the RWQM can provide valuable insights into zooplankton dynamics in the Bay, but it has had minimal validation of the zooplankton component. In particular, zooplankton biomass data— along a nutrient gradient—are critical for model assessment. Currently there are no reliable zooplankton biomass estimates, as the few estimates thus far have been taken with large mesh sizes that miss much of the zooplankton. Once the RWQM data have been validated, they could then provide the spatial and temporal extrapolation needed to understand Bay-wide zooplankton dynamics; something not possible to extract from site-specific field sampling data.
Note
We follow World Register of Marine Species, and the classifications contained here are correct at the time of publication.
Acknowledgements
Data were sourced from the Integrated Marine Observing System (IMOS)—a national collaborative research infrastructure system supported by the Australian Government. The Ecosystem Health Monitoring Program, Healthy Land and Water kindly provided jellyfish data.
References
-
- Schminke HK. 2007. Entomology for the copepodologist. Journal of Plankton Research. 29(1):i149-i162. http://dx.doi.org/10.1093/plankt/fbl073
- Condon RH, Steinberg DK, Bronk DA. 2010. Production of dissolved organic matter and inorganic nutrients by gelatinous zooplankton in the York River Estuary, Chesapeake Bay. Journal of Plankton Research. 32(2):153-170. http://dx.doi.org/10.1093/plankt/fbp109
- Frangoulis C, Skliris N, Lepoint G, Elkalay K, Goffart A, Pinnegar JK, Hecq J-H. 2011. Importance of copepod carcasses versus faecal pellets in the upper water column of an oligotrophic area. Estuarine, Coastal and Shelf Science. 92(3):456-463. https://doi.org/10.1016/j.ecss.2011.02.005
- Tang KW. 2005. Copepods as microbial hotspots in the ocean: Effects of host feeding activities on attached bacteria. Aquatic Microbial Ecology. 38(1):31-40
- Tang KW, Freund CS, Schweitzer CL. 2006. Occurrence of copepod carcasses in the lower Chesapeake Bay and their decomposition by ambient microbes. Estuarine, Coastal and Shelf Science. 68(3):499-508. https://doi.org/10.1016/j.ecss.2006.02.021
- Bishop JW, Greenwood JG. 1994. Nitrogen excretion by some demersal macrozooplankton in Heron and One Tree Reefs, Great Barrier Reef, Australia. Marine Biology. 120(3):447-453
- Marnane MJ, Bellwood DR. 2002. Diet and nocturnal foraging in cardinalfishes (Apogonidae) at One Tree Reef, Great Barrier Reef, Australia. Marine Ecology Progress Series. 231:261-268
- Holzman R, Genin A. 2003. Zooplanktivory by a nocturnal coral-reef fish: Effects of light, flow, and prey density. Limnology and Oceanography. 48(4):1367-1375. http://dx.doi.org/10.4319/lo.2003.48.4.1367
- Calbet A, Landry MR. 2004. Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnology and Oceanography. 49(1):51-57. http://dx.doi.org/10.4319/lo.2004.49.1.0051
- Leising AW, Horner R, Pierson JJ, Postel J, Halsband-Lenk C. 2005. The balance between microzooplankton grazing and phytoplankton growth in a highly productive estuarine fjord. Progress in Oceanography. 67(3-4):366-383. http://dx.doi.org/10.1016/j.pocean.2005.09.007
- Calbet A, Trepat I, Almeda R, Salo V, Saiz E, Movilla JI, Alcaraz M, Yebra L, Simo R. 2008. Impact of micro- and nanograzers on phytoplankton assessed by standard and size-fractionated dilution grazing experiments. Aquatic Microbial Ecology. 50(2):145-156. http://dx.doi.org/10.3354/ame01171
- Dinasquet J, Titelman J, Møller LF, Setälä O, Granhag L, Andersen T, Båmstedt U, Haraldsson M, Hosia A, Katajisto T. 2012. Cascading effects of the ctenophore Mnemiopsis leidyi on the planktonic food web in a nutrient-limited estuarine system. Marine Ecology Progress Series. 460:49-61
- Stone JP, Steinberg DK. 2018. Influence of top-down control in the plankton food web on vertical carbon flux: A case study in the Chesapeake Bay. Journal of Experimental Marine Biology and Ecology. 498:16-24. https://doi.org/10.1016/j.jembe.2017.10.008
- Edwards M, Beaugrand G, Hays GC, Koslow JA, Richardson AJ. 2010. Multi-decadal oceanic ecological datasets and their application in marine policy and management. Trends in Ecology and Evolution. 25(10):602-610
- Laing J. 1938. Report on research expedition: Science Students’ Association University of Queensland 1938 expedition – the plankton. The University of Queensland. Brisbane.
- Munro ISR. 1940. Studies on the marine invertebrate fauna of Moreton Bay, Queensland [Honours Thesis]. University of Queensland. Brisbane
- Greenwood JG. 1998. Zooplankton of Moreton Bay: The hidden processors. In: Tibbetts IR, Hall NJ, Dennison WC (Eds). Moreton Bay and Catchment. School of Marine Science, Brisbane. p. 347-364
- Greenwood JG. 1999. Task plankton tropodynamics (PTD) phase 2 final report, South East Queensland water quality strategy.
- Greenwood JG, Greenwood J, Skilleter GA. 2002. Comparison of demersal zooplankton in regions with differing extractive-dredging history, in the subtropical Brisbane River estuary. Plankton Biology and Ecology. 49(1):17-26
- Hewson I, Fuhrman JA. 2004. Richness and diversity of bacterioplankton species along an estuarine gradient in Moreton Bay, Australia. Applied and Environmental Microbiology. 70(6):3425-3433
- Uribe-Palomino J, Pausina S, Gershwin L-A. 2018. Two new species of hydromedusae from Queensland, Australia (Hydrozoa, Leptothecata). ZooKeys. 783:17-36. https://doi.org/10.3897/zookeys.783.26862
- Mauchline J. 1998. Advances in marine biology: The biology of calanoid copepods. Academic Press. 0065-2881
- Greenwood JG. 1980. Composition and seasonal variations of zooplankton populations in Moreton Bay, Queensland. Proceedings of the Royal Society of Queensland. 91:85-103
- Greenwood JG. 1982. Dominance, frequency and species richness patterns in occurrences of calanoid copepods in Moreton Bay, Queensland. Hydrobiologia. 87(3):217-227
- Greenwood JG. 1981. Occurrences of congeneric pairs of Acartia and Pseudodiaptomus species (Copepoda, Calanoida) in Moreton Bay, Queensland. Estuarine Coastal and Shelf Science. 13(5):591-596
- Thwin S. 1972. Cyclopoid copepods from Moreton Bay [PhD Thesis]. The University of Queensland. Brisbane
- Huys R, Boxshall GA. 1991. Copepod evolution. The Ray Society, London, England
- Greenwood JG. 1973. Calanoid copepods of Moreton Bay: A taxonomic and ecological account [PhD Thesis]. University of Queensland. Brisbane
- O’Neil JM, Roman MR. 1994. Ingestion of the cyanobacterium Trichodesmium spp. by pelagic harpacticoid copepods Macrosetella, Miracia and Oculosetella. Hydrobiologia. 292(1):235-240. http://dx.doi.org/10.1007/bf00229946
- O’Neil JM. 1998. The colonial cyanobacterium Trichodesmium as a physical and nutritional substrate for the harpacticoid copepod Macrosetella gracilis. Journal of Plankton Research. 20(1):43-59. http://dx.doi.org/10.1093/plankt/20.1.43
- Capone DG, Zehr JP, Paerl HW, Bergman B, Carpenter EJ. 1997. Trichodesmium, a globally significant marine cyanobacterium. Science. 276(5316):1221-1229. http://dx.doi.org/10.1126/science.276.5316.1221
- Heil C, O’Donuhue M, Dennison W. 1998. Aspects of the winter phytoplankton community of Moreton Bay. In: Tibbetts IR, Hall NJ, Dennison WC (Eds). Moreton Bay and Catchment. School of Marine Science, Brisbane. p. 291-300
- Hopcroft RR, Roff JC. 1998. Production of tropical larvaceans in Kingston Harbour, Jamaica: Are we ignoring an important secondary producer? Journal of Plankton Research. 20(3):557-569
- Jaspers C, Nielsen TG, Carstensen J, Hopcroft RR, Møller EF. 2009. Metazooplankton distribution across the southern Indian Ocean with emphasis on the role of larvaceans. Journal of Plankton Research. 31(5):525-540. http://dx.doi.org/10.1093/plankt/fbp002
- Alldredge AL. 1977. House morphology and mechanisms of feeding in the Oikopleuridae (Tunicata, Appendicularia). Journal of Zoology. 181:175-188
- Sato R, Tanaka Y, Ishimaru T. 2003. Species-specific house productivity of appendicularians. Marine Ecology Progress Series. 259:163-172
- Kiørboe T. 2001. Formation and fate of marine snow: Small-scale processes with large-scale implications. Scientia Marina. 65(S2):57-71
- Nishibe Y, Takahashi K, Ichikawa T, Hidaka K, Kurogi H, Segawa K, Saito H. 2015. Degradation of discarded appendicularian houses by oncaeid copepods. Limnology and Oceanography. 60(3):967-976. http://dx.doi.org/10.1002/lno.10061
- Alldredge AL. 1972. Abandoned larvacean houses: A unique food source in the pelagic environment. Science. 177(4052):885-887. http://dx.doi.org/10.1126/science.177.4052.885
- Katechakis A, Stibor H, Sommer U, Hansen T. 2004. Feeding selectivities and food niche separation of Acartia clausi, Penilia avirostris (Crustacea) and Doliolum denticulatum (Thaliacea) in Blanes Bay (Catalan Sea, NW Mediterranean). Journal of Plankton Research. 26(6):589-603. http://dx.doi.org/10.1093/plankt/fbh062
- Atienza D, Saiz E, Calbet A. 2006. Feeding ecology of the marine cladoceran Penilia avirostris: Natural diet, prey selectivity and daily ration. Marine Ecology Progress Series. 315:211-220
- Atienza D, Calbet A, Saiz E, Lopes RM. 2007. Ecological success of the cladoceran Penilia avirostris in the marine environment: Feeding performance, gross growth efficiencies and life history. Marine Biology. 151(4):1385-1396. http://dx.doi.org/10.1007/s00227-006-0578-8
- Kehayias G, Michaloudi E, Koutrakis E. 2005. Feeding and predation impact of chaetognaths in the north Aegean Sea (Strymonikos and Lerissos Gulfs). Journal of the Marine Biological Association of the United Kingdom. 85(6):1525-1532. http://dx.doi.org/10.1017/s0025315405012737
- Ojaveer H, Lankov A, Teder M, Simm M, Klais R. 2017. Feeding patterns of dominating small pelagic fish in the Gulf of Riga, Baltic Sea. Hydrobiologia. 792(1):331-344. http://dx.doi.org/10.1007/s10750-016-3071-5
- Rose K, Roff JC, Hopcroft RR. 2004. Production of Penilia avirostris in Kingston Harbour, Jamaica. Journal of Plankton Research. 26(6):605-615. http://dx.doi.org/10.1093/plankt/fbh059
- Bone Q, Kapp H, Pierrot-Bults AC. 1991. Introduction and relationships of the group. In: Bone Q, Kapp H, Pierrot-Bults AC (Eds). The biology of chaetognaths. Oxford University Press, Oxford, UK. p. 1-4
- Kehayias G, Kourouvakalis D. 2010. Diel vertical migration and feeding of chaetognaths in coastal waters of the eastern Mediterranean. Biologia. 65(2):301-308
- Balqis ARS, Yusoff FM, Arshad A, Nishikawa J. 2016. Seasonal variations of zooplankton biomass and size-fractionated abundance in relation to environmental changes in a tropical mangrove estuary in the Straits of Malacca. Journal of Environmental Biology. 37(4):685-695
- Kosobokova KN, Hirche H-J. 2016. A seasonal comparison of zooplankton communities in the Kara Sea – with special emphasis on overwintering traits. Estuarine, Coastal and Shelf Science. 175:146-156
- Payne JC. 1960. Scyphomedusae of northern and eastern Australian waters and from New Guinea [Honours Thesis]. University of Queensland. Brisbane
- Hamond R. 1971. Some medusae from near Brisbane. Search. 2(1):27
- Gorman NK. 1988. Planktonic Cnidaria and Ctenophora of Moreton Bay [Masters Thesis]. University of Queensland. Brisbane
- Davie P. 1998. Wild guide to Moreton Bay: Wildlife and habitats of a beautiful Australian coast – Noosa to the Tweed. Queensland Museum, Brisbane. 0724280529
- Gershwin L-A, Zeidler W, Davie PJ. 2010. Medusae (Cnidaria) of Moreton Bay, Queensland, Australia. Memoirs of the Queensland Museum. 54(3):47-108
- Tafe DJ. 1995. Cumacea (Crustacea: Peracarida) of Moreton Bay, Queensland: Taxonomy and ecology [PhD Thesis]. University of Queensland. Brisbane
- Joensen B. 2014. Identifying the spatio-temporal drivers of population dynamics for the jellyfish Catostylus mosaicus (Scyphozoa: Rhizostomeae) in Moreton Bay, Qld [Honours Thesis]. Griffith University. Brisbane
- Duggan S, McKinnon AD, Carleton JH. 2008. Zooplankton in an Australian tropical estuary. Estuaries and Coasts. 31(2):455-467. http://dx.doi.org/10.1007/s12237-007-9011-x
- Jacoby CA, Greenwood JG. 1989. Emergent zooplankton in Moreton Bay, Queensland, Australia: Seasonal, lunar, and diel patterns in emergence and distribution with respect to substrata. Marine Ecology Progress Series. 15(1-2):131-154
- Fancett MS, Kimmerer WJ. 1985. Vertical migration of the demersal copepod Pseudodiaptomus as a means of predator avoidance. Journal of Experimental Marine Biology and Ecology. 88(1):31-43. http://dx.doi.org/10.1016/0022-0981(85)90199-6
- Grindley J. 1972. The vertical migration behaviour of estuarine plankton. African Zoology. 7(1):13-20
- Jacoby CA, Greenwood JG. 1988. Spatial, temporal, and behavioral patterns in emergence of zooplankton in the lagoon of Heron Reef, Great Barrier Reef, Australia. Marine Biology. 97(3):309-328
- Lockington JR, Albert S, Fisher PL, Gibbes BR, Maxwell PS, Grinham AR. 2017. Dramatic increase in mud distribution across a large sub-tropical embayment, Moreton Bay, Australia. Marine Pollution Bulletin. 116(1):491-497. https://doi.org/10.1016/j.marpolbul.2016.12.029
- Coates-Marnane J, Olley J, Burton J, Sharma A. 2016. Catchment clearing accelerates the infilling of a shallow subtropical bay in east coast Australia. Estuarine, Coastal and Shelf Science. 174(Supplement C):27-40. https://doi.org/10.1016/j.ecss.2016.03.006
- Xiao Y, Greenwood JG. 1992. Distribution and behavior of Acetes sibogae Hansen (Decapoda, Crustacea) in an estuary in relation to tidal and diel environmental changes. Journal of Plankton Research. 14(3):393-407
- Sumpton W, Greenwood JG. 1990. Pre- and post-flood feeding ecology of four species of juvenile fish from the Logan-Albert estuarine system, Moreton Bay, Queensland. Australian Journal of Marine and Freshwater Research. 41(6):795-806
- Jacoby CA, Youngbluth MJ. 1983. Mating behavior in three species of Pseudodiaptomus (Copepoda: Calanoida). Marine Biology. 76(1):77-86
- Carr EF, Pitt KA. 2008. Behavioural responses of zooplankton to the presence of predatory jellyfish. Journal of Experimental Marine Biology and Ecology. 354(1):101-110.
https://doi.org/10.1016/j.jembe.2007.10.012
- Nagelkerken I, Pitt KA, Rutte MD, Geertsma RC. 2016. Ocean acidification alters fish -jellyfish symbiosis. Proceedings of the Royal Society B-Biological Sciences. 283(1833):7. http://dx.doi.org/10.1098/rspb.2016.1146
- Condon RH, Duarte CM, Pitt KA, Robinson KL, Lucas CH, Sutherland KR, Mianzan HW, Bogeberg M, Purcell JE, Decker MB, Uye S-i, Madin LP, Brodeur RD, Haddock SHD, Malej A, Parry GD, Eriksen E, Quinones J, Acha M, Harvey M, Arthur JM, Graham WM. 2013. Recurrent jellyfish blooms are a consequence of global oscillations. Proceedings of the National Academy of Sciences. 110(3):1000-1005. http://dx.doi.org/10.1073/pnas.1210920110
- Pitt KA, Kingsford MJ, Rissik D, Koop K. 2007. Jellyfish modify the response of planktonic assemblages to nutrient pulses. Marine Ecology Progress Series. 351:1-13. http://dx.doi.org/10.3354/meps07298
- West EJ, Pitt KA, Welsh DT, Koop K, Rissik D. 2009. Top‐down and bottom‐up influences of jellyfish on primary productivity and planktonic assemblages. Limnology and Oceanography. 54(6):2058-2071
- Lamb PD, Hunter E, Pinnegar JK, Creer S, Davies RG, Taylor MI. 2017. Jellyfish on the menu: mtDNA assay reveals scyphozoan predation in the Irish Sea. Royal Society Open Science. 4(11):171421
- Welsh DT, Dunn RJ, Meziane T. 2009. Oxygen and nutrient dynamics of the upside down jellyfish (Cassiopea sp.) and its influence on benthic nutrient exchanges and primary production. Hydrobiologia. 635(1):351-362
- Browne JG, Kingsford MJ. 2005. A commensal relationship between the scyphozoan medusae Catostylus mosaicus and the copepod Paramacrochiron maximum. Marine Biology. 146(6):1157-1168
- Browne JG, Pitt KA, Norman MD. 2017. Temporal patterns of association between the jellyfish Catostylus mosaicus and a sphaeromatid isopod and parasitic anemone. Marine and Freshwater Research. 68(9):1771-1777. https://doi.org/10.1071/MF16076
- Nagelkerken I, Pitt KA, Rutte MD, Geertsma RC. 2016. Ocean acidification alters fish–jellyfish symbiosis. Proceedings of the Royal Society B. 283(1833):20161146
- Browne JG. 2015. Parasites of jellyfish in eastern Australia [PhD Thesis]. Griffith University. Brisbane
- Pitt KA, Welsh DT, Condon RH. 2009. Influence of jellyfish blooms on carbon, nitrogen and phosphorus cycling and plankton production. Hydrobiologia. 616:133-149. https://doi.org/10.1007/s10750-008-9584-9
- Chelsky A, Pitt KA, Ferguson AJ, Bennett WW, Teasdale PR, Welsh DT. 2016. Decomposition of jellyfish carrion in situ: Short-term impacts on infauna, benthic nutrient fluxes and sediment redox conditions. Science of the Total Environment. 566:929-937
- Pitt KA, Welsh DT, Condon RH. 2009. Influence of jellyfish blooms on carbon, nitrogen and phosphorus cycling and plankton production. Hydrobiologia. 616(1):133-149
- Pitt KA, Koop K, Rissik D. 2005. Contrasting contributions to inorganic nutrient recycling by the co-occurring jellyfishes, Catostylus mosaicus and Phyllorhiza punctata (Scyphozoa, Rhizostomeae). Journal of Experimental Marine Biology and Ecology. 315:71-86
- Liu H, Zhang X, Yang Q, Zuo T, Quigg A. 2017. Mesozooplankton dynamics in relation to environmental factors and juvenile fish in a subtropical estuary of the Gulf of Mexico. Journal of Coastal Research. 33(5):1038-1050
- Ke Z, Tan Y, Huang L, Liu J, Liu H. 2018. Community structure and biovolume size spectra of mesozooplankton in the Pearl River Estuary. Aquatic Ecosystem Health & Management. 21(1):30-40. http://dx.doi.org/10.1080/14634988.2018.1432948
- Uye S-I. 1994. Replacement of large copepods by small ones with eutrophication of embayments; cause and consequence. Hydrobiologia. 292-293(0):513-519
- Park GS, Marshall HG. 2000. Estuarine relationships between zooplankton community structure and trophic gradients. Journal of Plankton Research. 22(1):121-136. http://dx.doi.org/10.1093/plankt/22.1.121
- Dufrêne M, Legendre P. 1997. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological Monographs. 67(3):345-366
- Hopcroft RR, Roff JC, Lombard D. 1998. Production of tropical copepods in Kingston Harbour, Jamaica: The importance of small species. Marine Biology. 130(4):593-604
- Calbet A, Landry MR, Scheinberg RD. 2000. Copepod grazing in a subtropical bay: Species-specific responses to a midsummer increase in nanoplankton standing stock. Marine Ecology Progress Series. 193:75-84
- Lonsdale DJ, Coull BC. 1977. Composition and seasonality of zooplankton of North Inlet, South Carolina. Chesapeake Science. 18(3):272-283
- Chew L-L, Chong VC. 2011. Copepod community structure and abundance in a tropical mangrove estuary, with comparisons to coastal waters. Hydrobiologia. 666(1):127-143. http://dx.doi.org/10.1007/s10750-010-0092-3
- Lopes RM, Brandini FP, Gaeta SA. 1999. Distribution patterns of epipelagic copepods off Rio de Janeiro (SE Brazil) in summer 1991/1992 and winter 1992. Hydrobiologia. 411:161-174
- Kimmerer WJ, McKinnon AD. 1985. A comparative study of the zooplankton in two adjacent embayments, Port Phillip and Westernport Bays, Australia. Estuarine Coastal and Shelf Science. 21(2):145-159
- Herzfeld M, Jones E, Margvelashvili N, Mongin M, Skerratt J, Andrewartha J, Rizwi F, McAlister T, Holmes R, Barry M, Weber T, Teakle I, Baird M. 2014. SEQ RWQM V3 Phase II final report with biogeochemical modelling (Ch 4) update. CSIRO. DOI: https://doi.org/10.4225/08/596671e8a721f.
- Bayly IAE. 1965. Ecological studies on the planktonic copepoda of the Brisbane River estuary with special reference to Gladioferens pectinatus (Brady) (Calanoidea). Australian Journal of Marine and Freshwater Research. 16(3):315-350
- Greenwood JG. 1976. Calanoid copepods of Moreton Bay (Queensland) I. Families Calanidae, Eucalanidae and Paracalanidae. Proceedings of the Royal Society of Queensland. 87:1-28
- Greenwood JG. 1977. Calanoid copepods of Moreton Bay (Queensland) II. Families Calocalanidae to Centropagidae. Proceedings of the Royal Society of Queensland. 88:49-67
- Greenwood JG. 1978. Calanoid copepods of Moreton Bay (Queensland) III. Temoridae to Tortanidae excluding Pontellidae. Proceedings of the Royal Society of Queensland. 89:1-21
- Greenwood JG. 1979. Calanoid copepods of Moreton Bay (Queensland) IV. Family Pontellidae. Proceedings of the Royal Society of Queensland. 90:93-111
- Bayly IAE, Greenwood JG. A new species of Calanopia (Copepoda: Calanoida) from Moreton Bay, Queensland. Proceedings of the Royal Society of Queensland. 77(11):99-105
- Greenwood JG. 1982 Calanoid copepods of Moreton Bay (Queensland) V. Ecology of the dominant species. Proceedings of the Royal Society of Queensland. 93:49-64
- Moore SK, Suthers IM. 2006. Evaluation and correction of subresolved particles by the optical plankton counter in three Australian estuaries with pristine to highly modified catchments. Journal of Geophysical Research: Oceans. 111(C5)
- Gismervik I, Olsen Y, Vadstein O. 2002. Micro- and mesozooplankton response to enhanced nutrient input – a mesocosm study. Hydrobiologia. 484(1-3):75-87
Mouillot D, Spatharis S, Reizopoulou S, Laugier T, Sabetta L, Basset A, Chi TD. 2006. Alternatives to taxonomic-based approaches to assess changes in transitional water communities. Aquatic Conservation: Marine and Freshwater Ecosystems. 16(5):469-482. http://dx.doi.org/10.1002/aqc.769






