-
Previous paper
Ecology of the marine mammals of Moreton Bay
Janet M Lanyon1, Michael J Noad2, Justin Meager3 -
This paper
Migratory shorebirds of Moreton Bay
Richard Fuller1, David A. Milton2,3†, Peter Rothlisberg2,3, Robert S. Clemens1, Jon Coleman2, Kristy Murray4, Kiran L. Dhanjal-Adams5, David Edwards2, Paul G. Finn2, Greg Skilleter1, Madeleine Stigner1 and Bradley K. Woodworth1 -
Next paper
How does citizen science contribute to sustaining Moreton Bay? A discussion of approaches and applications.
Jennifer Loder1,2, Chris Roelfsema1,3, Carley Kilpatrick4, Victoria Martin2,5
Migratory shorebirds of Moreton Bay
Authors
Richard Fuller1, David A. Milton2,3†, Peter Rothlisberg2,3, Robert S. Clemens1, Jon Coleman2, Kristy Murray4, Kiran L. Dhanjal-Adams5, David Edwards2, Paul G. Finn2, Greg Skilleter1, Madeleine Stigner1 and Bradley K. Woodworth1Author affiliations
- School of Biological Sciences, University of Queensland, St Lucia Qld, 4072, Australia
- Queensland Wader Study Group, Brisbane, Australia
- CSIRO Oceans and Atmosphere, PO Box 2582, Brisbane Qld 4001, Australia
- Department of Environment and Science, GPO Box 2454 Queensland 4001
- Swiss Ornithological Institute, Seerose 1, Sempach CH-6204, Luzern, Switzerland.
†David tragically passed away during the preparation of this manuscript, and we dedicate it to his memory.
Corresponding author
r.fuller@uq.edu.auORCID
Richard A. Fuller: https://orcid.org/0000-0001-9468-9678
Peter Rothlisberg: https://orcid.org/0000-0001-5632-5996
Robert S. Clemens: https://orcid.org/0000-0002-1359-5133
Kiran L. Dhanjal-Adams: https://orcid.org/0000-0002-0496-8428
Greg Skilleter: https://orcid.org/0000-0002-4719-0409
Madeleine Stigner: https://orcid.org/0000-0002-9393-734X
Bradley K. Woodworth: https://orcid.org/0000-0002-4528-8250
Book
Migratory shorebirds of Moreton Bay
Chapter
Research Paper Title
Migratory shorebirds of Moreton Bay
Cite this paper as:
Fuller R, Milton DA, Rothlisberg P, Clemens RS, Coleman J, Murray K, Dhanjal-Adams KL, Edwards D, Finn PG, Skilleter G, Stigner M. Woodworth BK. 2019. Migratory shorebirds of Moreton Bay. In Tibbetts, I.R., Rothlisberg, P.C., Neil, D.T., Homburg, T.A., Brewer, D.T., & Arthington, A.H. (Editors). Moreton Bay Quandamooka & Catchment: Past, present, and future. The Moreton Bay Foundation. Brisbane, Australia. Available from: https://moretonbayfoundation.org/
DOI
10.6084/m9.figshare.8074379
ISBN
978-0-6486690-0-5
Abstract
Tens of thousands of migratory shorebirds return to Moreton Bay each year from their breeding grounds in the Arctic. The Bay’s extensive tidal flats provide a rich feeding resource for the birds while they recuperate from their long migration flight and prepare for their next one. The abundance of many migratory shorebird species has declined dramatically in Moreton Bay, and while some of the causes are located elsewhere along the birds’ migration routes, there are significant threats to the birds and their habitats within the Bay, ranging from habitat loss to disturbance. New partnerships between conservation management agencies and NGOs have led to exciting examples of conservation action to reduce some of these threats, including collecting high quality monitoring data, careful zoning of recreational and commercial uses to avoid important areas for shorebirds, and extensive awareness-raising activities. Migratory shorebird conservation will become more and more critical as the human population using the Bay continues to increase over the coming decades.
Keywords: birds, population declines, monitoring, zoning, marine park
Introduction
Migratory shorebirds undertake some of the longest regular migrations of any animal group, with many species breeding in the high Arctic tundra and migrating all the way to the Southern Hemisphere to spend the non-breeding season, often stopping along the way to refuel in the vast tidal flats of East Asia (Fig. 1). Small tags fitted to the birds have revealed the magnitude of the journeys they undertake, and the bar-tailed godwit, Limosa lapponica, is one of the best studied species (1–3). The subspecies baueri flies from eastern Australia north to the eastern coast of the Yellow Sea to refuel (2, 3), then on to Alaska to breed. Following breeding, it flies non-stop across the Pacific Ocean in a flight of almost 12,000 km to its non-breeding grounds in eastern Australia and New Zealand (4).
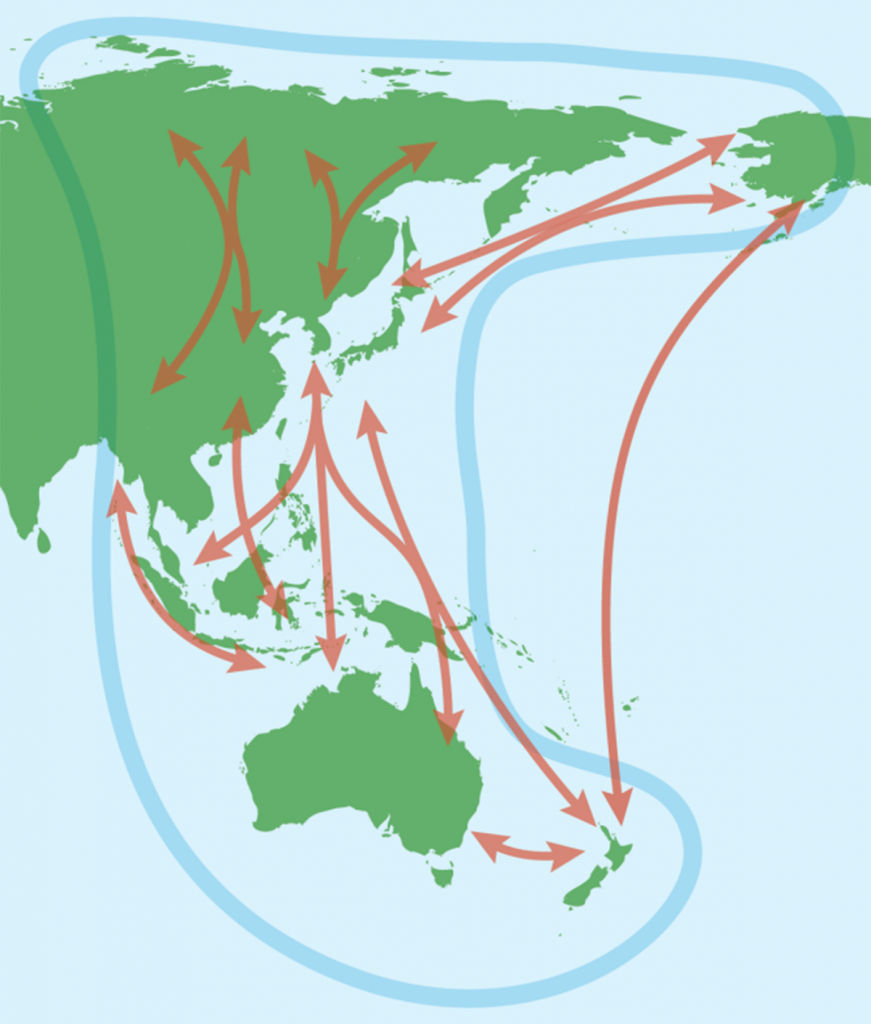
What does all this mean for Moreton Bay? Moreton Bay is the crucial end point of the journey of the bar-tailed godwit and a number of other species. Moreton Bay has supported up to 33,900 migratory shorebirds annually in the period since 2011 (Table 1). It is one of the most numerically important non-breeding sites for shorebirds in Australia, and supports internationally significant numbers (>1% of the total flyway population) of at least nine migratory shorebird species (Table 1) (5, 6). In 1993, it was declared both an internationally important wetland under the Ramsar Convention on Wetlands and a marine park, partly on the basis of the bird populations. A zoning plan providing for the ecologically sustainable use of the park was produced in 1997 (7).
Ecologically sustainable use is of course critical for the long-term health of migratory shorebird populations in Moreton Bay, where birds are continually affected by habitat loss and disturbance (8, 9). Coastal ecosystems in Moreton Bay are critical for providing food and shelter for the shorebirds to recover from their long migration, conduct a feather moult, and gain condition again before the next long migration back to the breeding grounds. Such migrations may seem extreme, but the birds are uniquely adapted to undertake the journeys (10), which allow them to exploit a summer flush of resources in the Arctic, and spend the non-breeding season feeding on the rich benthic infauna of sediments in estuaries such as Moreton Bay. Yet many migratory shorebird species are in rapid decline in the East Asian–Australasian Flyway (11–13). In Moreton Bay, at least six species of migratory shorebird were identified as in rapid decline in an analysis of monitoring data collected by the Queensland Wader Study Group (QWSG) between 1992 and 2008 (red knot, bar-tailed godwit, ruddy turnstone, common greenshank, great knot and whimbrel), and a further two were possibly in decline (greater sand plover, far eastern curlew) (14).
The declines are thought to be mostly driven by habitat loss in the East-Asian stopover areas where, for example, more than two-thirds of intertidal habitat has been lost in the Yellow Sea in the last 50 years, primarily as a result of land reclamation for infrastructure development (15). Indeed, recent studies have shown that the Australian species declining most quickly are those that are highly dependent on the Yellow Sea while on migration (13), and that survival rates are declining for migratory shorebirds that depend on the Yellow Sea (16). Yet migratory species depend on a complete chain of intact habitats along their migration routes (17), and habitat degradation anywhere along the chain can impact the birds (18). Thus, the proper management of important sites such as Moreton Bay is crucial in the context of the birds’ lengthy migration journeys.
Table 1. Species and highest count of migratory shorebirds estimated in Moreton Bay regularly since 2008 (61). Data are extracted from the Queensland Wader Study Group monitoring database. Note that all migratory shorebirds are listed as migratory/marine under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) — the conservation listings shown here relate to listings as threatened species only.
| Species | Count | Internationally significant numbers | Conservation listing (EPBC Act) |
|---|---|---|---|
| Asian dowitcher | < 20 | Not listed | |
| Bar-tailed godwit | 11650 | Yes | Vulnerable |
| Black-tailed godwit | 694 | Not listed | |
| Broad-billed sandpiper | 131 | Not listed | |
| Common greenshank | 187 | Not listed | |
| Common sandpiper | < 20 | Not listed | |
| Curlew sandpiper | 2126 | Yes | Critically Endangered |
| Double-banded plover | 307 | Not listed | |
| Far eastern curlew | 3158 | Yes | Critically Endangered |
| Great knot | 1433 | Critically Endangered | |
| Greater sand plover | 187 | Vulnerable | |
| Grey plover | 57 | Not listed | |
| Grey-tailed tattler | 2430 | Yes | Not listed |
| Latham's snipe | < 20 | Not listed | |
| Lesser sand plover | 1949 | Yes | Endangered |
| Marsh sandpiper | 125 | Not listed | |
| Pacific golden plover | 739 | Yes | Not listed |
| Red knot | 1044 | Endangered | |
| Red-necked stint | 4919 | Yes | Not listed |
| Ruddy turnstone | 160 | Not listed | |
| Sanderling | 6 | Not listed | |
| Sharp-tailed sandpiper | 1550 | Not listed | |
| Terek sandpiper | 195 | Not listed | |
| Wandering tattler | < 20 | Not listed | |
| Whimbrel | 1140 | Yes | Not listed |
Status and ecology of migratory shorebirds in Moreton Bay
About 23 species of migratory shorebird regularly occur in Moreton Bay, with another five migratory species recorded irregularly along with another 13 locally breeding, non-migratory species of shorebird. The most abundant migratory shorebird is the bar-tailed godwit, followed by the red-necked stint. Moreton Bay also supports all the threatened species of migratory shorebird listed under the federal Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) (Table 1). It has one of the largest populations of the ‘Critically Endangered’ far eastern curlew in Australia, and is now one of the last major strongholds in the world for this rapidly declining species.

Non-breeding shorebirds typically feed on invertebrates that live in or on intertidal habitats, mostly within soft sand and mud in Moreton Bay (19, 20). As the tide comes in and covers feeding habitats, birds move to high-tide roost areas, where they often gather together in large numbers to rest, preen and sleep, and supplement their feeding if the opportunity arises. High-tide roosts are often in claypans, rocky headlands, mangroves, or a range of artificial sites where they can roost away from disturbance and where they have good visibility of the surrounding area to scan for approaching predators (21, 22). This dependence on two markedly different kinds of habitat every day is a crucial factor for understanding the ecology and conservation management needs of shorebirds. To occupy an area, shorebirds need access to high-quality feeding sites, but also nearby suitable roosting sites (21, 23). Energy reserves can be conserved by minimising the flight distance between roosting and feeding areas. For far eastern curlew in Moreton Bay, the typical distance travelled between roosts and feeding grounds is 5 to 10 km (23). As the mainland coast of Moreton Bay has become increasingly developed, there are now few places where significant numbers of birds can gather to roost free of disturbance, and disturbance of roosting birds is a significant management challenge (Fig. 2) (24).
Migratory shorebird monitoring in Moreton Bay
Migratory shorebirds in Moreton Bay have been systematically counted by volunteers at up to 180 coastal sites from 1992 onward (25, Box 1). Monthly counts are conducted around high tide (80% of visits made within 2 hours of the time of high tide), when birds are concentrated at roost sites (22). The number of sites visited per year increased between 1992 and 1995 and has remained relatively stable thereafter. The spatial extent of survey effort was also greater in summer months (January–March) when non-breeding migrants are most abundant.
Box 1. Queensland Wader Study Group shorebird monitoring
The Queensland Wader Study Group, a special-interest group of Birds Queensland, was established in 1992 to monitor and conserve shorebird populations. Run entirely by volunteers (like most shorebird monitoring in Australia), close interaction between organisers and surveyors has been key to the accuracy, precision, coverage and longevity of shorebird monitoring in Queensland. Unlike any other regional shorebird- monitoring effort around the nation, one notable feature of monitoring in parts of Queensland is monthly counting, which reduces within-year count variability and increases statistical power to detect trends compared with less frequent monitoring elsewhere.
Habitats for migratory shorebirds in Moreton Bay
(i) Intertidal soft sediment and hard substrates
Moreton Bay contains a complex system of intertidal flats totalling some 23,000 ha at low tide (26), providing a range of feeding habitats for shorebirds. Substrate types within the Bay are diverse and have been broadly categorised into sand, coral, sandy-mud and mud (27). Sand is more prevalent in the eastern side of the Bay and tends to be more penetrable than mud or sandy-mud. This is because the latter frequently contains higher proportions of resistant material such as rocks, coral or shells just below the surface (19). Therefore, there is a need to look below the substrate surface to assess the suitability of feeding habitat for deep-probing shorebirds. Substrate penetrability has been shown to be a good predictor of far eastern curlew feeding density at the broad scale within Moreton Bay, with lower densities of far eastern curlew in areas where the substrate has a low penetrability (19). This is hardly surprising when one considers that the bird can rapidly thrust its whole head into the substrate, reaching a depth of over 20 cm, to capture large, deep-burrowing crustaceans. Pressure-sensitive receptors in the bill of some probing shorebirds allow them to detect solid objects embedded in the wet substrate (28), but inanimate objects buried within the substrate could also interfere with prey detection and capture, and even damage the birds’ bills. Several shorebird species have been shown to switch between tactile hunting on soft substrates and visual hunting on hard substrates (e.g. 29, 30). In terms of assessing and monitoring the quality of feeding grounds for deep-probing shorebirds, a simple measure of substrate penetrability would be the most efficient method, and it could be used to map their probability of use across landscapes.
(ii) Supra-tidal, mangroves, saltmarshes, artificial habitats as roosting and supplementary feeding sites
A subset of the shorebird species that spend their non-breeding season in Moreton Bay has a strong affinity with mangroves for roosting and sometimes feeding. There are three main species that associate regularly with mangroves for roosting. These are grey-tailed tattler, Terek sandpiper and whimbrel. Whimbrel also feed close to and among mangroves in many parts of Moreton Bay, such as Pumicestone Passage and the southern Bay islands.
The main artificial habitats in Moreton Bay used by shorebirds are in the Port of Brisbane reclamation area at the mouth of the Brisbane River. Bunded ponds in various stages of partial reclamation provide extensive roosting habitat adjacent to the rich intertidal feeding grounds at the mouth of the river. These reclamation ponds also provide non-tidal feeding habitat for the smaller migratory shorebirds such as curlew sandpiper and red-necked stint. During the early stages of reclamation, sediment from dredging of the main shipping channel is pumped into the ponds. These sediments contain small invertebrates, including bivalves and crustaceans, that are prey of small shorebirds. The provision of these additional feeding opportunities has led to an increase in the overall Moreton Bay population of red-necked stint and their concentration within the Port of Brisbane reclamation area. It is unclear how these shorebirds will respond when the reclamation is complete and the additional artificial feeding habitat is lost.
Threats to migratory shorebirds in Moreton Bay
There are numerous threats to migratory shorebird populations in Moreton Bay, and more generally in the flyway, including climate change, which may affect wetland breeding habitat in the Arctic (31); loss of stopover sites in mainland Asia (15, 32, 33), and reduction in the area and quality of non-breeding grounds, primarily in Australia (34).
In Moreton Bay, far eastern curlews require deep, soft sediment to be able to use their extremely long bill to its full potential and achieve their greatest foraging success (20). Any structural modification of soft-sediment feeding flats that reduces substrate penetrability may inhibit successful foraging and be detrimental to deep-probing shorebirds (20). Direct and indirect effects on the structure of soft sediments could come from activities including intertidal oyster farming, bait harvesting, the compaction of sediments by vehicles, beach nourishment, nutrient enrichment and the dumping of rubbish or debris (35).
Additional threats include loss of habitat through development, changes in benthic food availability, changes in mangrove and seagrass distribution, and human disturbance.
Management of migratory shorebirds in Moreton Bay
The commitment of Australian governments to protect shorebirds in Australia is reflected in federal and state legislation such as the EPBC Act 1999, Nature Conservation Act 1992 and Marine Parks Act 2004. Such legislation provides for the listing of shorebird species, declaration of marine and terrestrial protected areas, development of recovery plans, and assessment of actions that may impact shorebirds or their habitat.
Development and land-use planning in the coastal zone managed under the Coastal Protection and Management Act 1995 and state planning policies provide protection and management of coastal resources and link to matters of state interest such as marine park highly protected areas, ecologically significant wetlands and wildlife habitat. Numerous other pieces of legislation exist to protect marine resources and habitat to the benefit of shorebirds such as the Environmental Protection Act 1994 and Fisheries Act 1994 and declared fish habitat areas.
The Moreton Bay Marine Park aims to conserve the unique values of Moreton Bay whilst allowing activities such as commercial and recreational use to occur. This balancing act is achieved through zoning that protects representative habitat types and regulates entry and use via the Marine Park (Moreton Bay) Zoning Plan 2008. Protecting sensitive habitats and species is a key consideration in administering the marine park and the zoning plan contains specific provisions intended to protect shorebirds and their habitat from unreasonable disturbance. Disturbance levels at shorebird roost sites in Moreton Bay are strongly related to marine park zones, with marine national park zones, the most highly protected zone, showing the lowest frequency of disturbance to shorebirds (36).
The Migratory Shorebird Conservation Action Plan and Shorebird Management Strategy of Moreton Bay provide guidance to cooperatively manage shorebirds. The Shorebird Management Strategy of Moreton Bay adopts a multifaceted approach to shorebird management, including protecting critical shorebird habitat, protecting shorebirds from disturbance and conducting research and monitoring. Practical mechanisms for achieving this include assessment and the placement of conditions on activities, compliance enforcement, education and awareness, regulation of access or activities, and cooperative management with local councils.
Active shorebird management in accordance with the above statutory and non-statutory tools depends on the responsibility and jurisdiction of relevant authorities. While there is a solid legislative basis on which to base and guide shorebird management, resources and funding allocation is a matter of competing priority within governments. Therefore, effective management is best achieved in collaboration with natural resource management bodies and non-profit organisations that are eligible to apply for grant funding.
Managing migratory shorebirds is challenging for three interlinked reasons:
(i) complex multi-uses of the landscape
Moreton Bay Marine Park is a multi-use marine reserve with areas designated as general use, habitat protection, conservation park and marine national park. Recreational and commercial access is allowed to most areas under the provision that shorebirds are not disturbed. Despite regulations against disturbance, the reality of allowing recreational activities on beaches means that birds are regularly in contact with kite surfers, horse riders, fishers, four-wheel-drive vehicles and dog walkers.
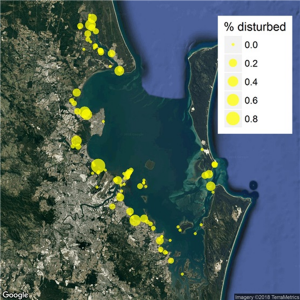
Human recreational use of natural areas can incur immediate behavioural costs to birds, including increased energy expenditure and loss of foraging time as a result of increased time spent being vigilant. In some cases, temporary or permanent avoidance of suitable habitat can occur, ultimately reflected in lower local abundance, poorer physiological condition or impaired reproductive success (8, 37). An analysis of QWSG data showed dogs, walkers and fishing to be the most frequent anthropogenic causes of disturbance to roosting shorebirds in Moreton Bay (36). Notwithstanding education, signage and enforcement of shorebird disturbance laws in the marine park, a high level of disturbance is still occurring at the majority of shorebird sites in the Moreton Bay Marine Park (Fig. 3).
Dog walking in particular can have a large impact on shorebirds, as many dogs are not kept on leashes on the beach (38). Furthermore, dogs actively and repeatedly chase shorebirds, forcing birds to either repeatedly take flight, to increase their vigilance, or even to leave an area. Disturbance by dogs is also a major issue in the terrestrial environment, and substantial reductions in woodland bird abundance have been documented as a result of dog disturbance, suggesting a need to restrict access by dogs in sensitive conservation areas (39).
Dogs must be kept under close control across the intertidal areas of Moreton Bay, yet the reality of living in a city means that beaches are one of the few places where dog owners can let their dogs run freely. Though common, disturbance by dogs is also one of the most easily manageable threats to shorebirds in Moreton Bay. There are two primary methods for managing disturbances to shorebirds: one is to manage public access to important shorebird areas; the other is to allow access to beaches while managing activities. The reality of restricting access to beaches in populated areas, however, means that large nature reserves such as Moreton Bay are difficult to enforce, particularly when management boundaries differ at the council, state and national levels, as is the case for intertidal habitats. Furthermore, restricting access creates conflict in highly populated areas.
In fact research shows that increasing access for people walking their dogs off-leash, and ensuring a smaller more restricted area important for shorebirds is better enforced (8, 24), can produce a win–win situation whereby important shorebird habitat is protected, and dog walkers have the area needed to exercise their dogs.
(ii) energetic consequences of disturbance
Long-distance migrations are energetically demanding, and shorebirds have developed a range of physiological adaptations enabling them to achieve such journeys. Prior to migration, shorebirds are able to increase their energy stores over very short periods through rapid weight gain of 50 to 80% of their body mass (40). To allow for this weight gain and for an increase in the size of flight muscles, birds must compensate through drastic shrinkage of certain organs (41, 42). During flight, energy consumption can remain relatively low and energy is burned straight from organs if needed (42). Refuelling prior, during and after migration is therefore essential in ensuring survival of the species. Repeated disturbance to shorebirds can prevent individuals from gaining the necessary weight to complete migration.
Shorebirds feed in the intertidal zone and roost during high tide, when large numbers concentrate into a small area. Disturbance during feeding can interrupt foraging, and disturbance during roosting can cause birds to take flight, wasting energy reserves. Indeed, shorebirds are highly responsive to anthropogenic stimuli and thus are readily disturbed (38). In the short-term, disturbance can result in increased levels of stress and behavioural changes (43). In the long-term, disturbance can result in chronic avoidance of disturbed habitat and abandonment of otherwise suitable habitat as individuals move to less-disturbed areas (44), increasing density and therefore competition between individuals at undisturbed sites (45).
Shorebirds can better conserve their energy at sites where there is little disturbance (46). The worst case is when birds are forced to stop feeding altogether or have to leave for a lower quality feeding area. Faster human movements (i.e. running as opposed to walking, jetskiing as opposed to canoeing) cause greater disturbance to shorebirds (47, 48). It is possible for shorebirds to adapt to human disturbance, by either extending their feeding period or by becoming habituated to the disturbance (49). It has been suggested that larger shorebirds may be less tolerant of human disturbance than smaller shorebirds (50, 51).
(iii) both local and remote drivers of change could be impacting the health of shorebird populations in Moreton Bay
Because many shorebird species are migratory, the number of birds we see in Moreton Bay can be influenced not only by conditions in Moreton Bay, but also by conditions hundreds, if not thousands, of kilometres away. Moreton Bay like much of Australia suffers from extreme rainfall and drought events, both of which can negatively impact shorebirds. The first by changing sediment structure and therefore food availability (52, 53), the latter by reducing inland habitat availability, thus forcing large numbers of birds to concentrate into a small number of coastal roosting sites and increasing their susceptibility to disturbance events. Repeated disturbances force birds to repeatedly take flight with nowhere else to go.
Though these impacts can be small in isolation, they can have a cumulative effect on birds by preventing them from gaining the necessary weight to start migration in an optimal body condition. As a result, migratory fitness can be reduced, and likewise migratory endurance and speed, meaning some birds will not be able to fly the required distances to reach their stopover sites spread across East Asia. Stopover sites are few and far between, making each individual site important for replenishing depleted fat reserves.
Within the East Asian–Australasian Flyway, the most strategically situated and nutritious stopover sites are located in the Yellow Sea. Most shorebirds funnel through this region on their way to the breeding grounds, creating a large migratory bottleneck (18). However, the Yellow Sea is one of the most populated regions in the world, and hunting, pollution, habitat loss and disturbance there are potentially impacting on the number of birds able to return to Australia each year (54). Loss of even small habitat patches in this location can result in disproportionately large contributions to shorebird declines (55).
Poor resting and refueling conditions in stopover sites mean birds continue their journey in poor physiological condition, and may not reach the breeding grounds in good enough condition to establish a good quality breeding territory. Shorebirds are primarily income breeders, meaning they gain some resources for breeding while in the breeding grounds (56). Conditions upon arrival are therefore important in determining the number of eggs and clutches produced, as well as chick survival. The Arctic is however one of the habitats most sensitive to climate change. Not only is the amount of available habitat predicted to decrease for shorebirds (57), but so is the timing of arctic green-up and peak abundance of arthropod prey, on which shorebirds depend while breeding. Indeed, warmer summers are predicted to cause an increase in mosquito growth rate, resulting in a shorter period where mosquitoes are available as a food source for shorebirds. A shorter season also means birds grow less and there is already evidence of red knots developing shorter bills (58). Once back in their tropical intertidal habitats, these birds with shorter bills have difficulties accessing their usual prey items buried in the mudflats, and suffer higher rates of mortality as a result.
Emerging issues
With the human population in the Greater Brisbane projected to grow rapidly in the coming decade (59), pressure on the natural environment of Moreton Bay looks set to intensify both in terms of recreational and commercial use, and major development projects. From the perspective of migratory shorebirds, we urge careful thought about the following five issues:
- Continued generation of high-quality monitoring data, ideally through monthly shorebird monitoring at the major sites throughout Moreton Bay, with comprehensive bay-wide counts at least quarterly.
- Integration of shorebird monitoring data into all relevant planning and decision-making tools within local and state government, together with continued updating of information.
- Use of decision-support tools to plan and enforce recreational zoning across Moreton Bay, ideally as a partnership between state government and all local government areas adjacent to Moreton Bay.
- Provision of data and availability of expertise to assist interpretation, supporting investigations of the impacts of proposed development projects.
- Periodic expert analysis of shorebird monitoring data to assess any impacts of Moreton Bay’s changing environment on bird numbers, and to identify success or failure of conservation efforts.
Moreton Bay is a critically important site for migratory shorebirds in the East Asian–Australasian Flyway. Enormous efforts have been made, and are being made, to protect its ecological integrity in the face of strong demand for recreational and commercial use. Continued proper management and protection of key habitats are paramount for its long-term future as a non-breeding destination for migratory shorebirds.
References
- Gill RE, Piersma T, Hufford G, Servranckx R, Riegen A. 2005. Crossing the ultimate ecological barrier: Evidence for an 11,000 km long nonstop flight from Alaska to New Zealand and eastern Australia by Bar-tailed Godwits. Condor. 107:1-20
- Wilson JR, Nebel S, Minton CDT. 2007. Migration ecology and morphometrics of two Bar-tailed Godwit populations in Australia. Emu. 107:262-274
- Battley PF, Warnock N, Tibbitts TL, Gill RE, Piersma T, Hassell CJ, Douglas DC, Mulcahy DM, Gartrell BD, Schuckard R, Melville DS. 2012. Contrasting extreme long-distance migration patterns in bar-tailed godwits Limosa lapponica. Journal of Avian Biology. 43:21-32
- Gill RE, Tibbitts TL, Douglas DC, Handel CM, Mulcahy DM, Gottschalck JC, Warnock N, McCaffery BJ, Battley PF, Piersma T. 2009. Extreme endurance flights by landbirds crossing the Pacific Ocean: Ecological corridor rather than barrier? Proceedings of the Royal Society B. 276:447-57
- Bamford M, Watkins D, Bancroft W, Tischler G, Wahl J. 2008. Migratory shorebirds of the East Asian–Australasian flyway: Population estimates and internationally important sites. Wetlands International–Oceania, Canberra
- Oldland J, Clemens R, Haslem A, Shelley L, Kearney B. Shorebirds 2020: Migratory shorebird population monitoring project. Final report to the Department of Environment, Water, Heritage and the Arts. Birds Australia, Carlton, Australia
- Queensland Government. 1997. Marine Parks (Moreton Bay) Zoning Plan 1997. Queensland Government, Brisbane
- Stigner MG, Beyer HL, Klein CJ, Fuller RA. 2016. Reconciling recreational use and conservation values in a coastal protected area. Journal of Applied Ecology. 53:1206-1214
- McPhee D. 2017. Environmental History and Ecology of Moreton Bay. CSIRO Publishing, Clayton, Victoria, Australia
- Geering A, Agnew L, Harding S. 2007. Shorebirds of Australia. CSIRO Publishing, Collingwood, Victoria
- Amano T, Székely T, Koyama K, Amano H, Sutherland WJ. 2010. A framework for monitoring the status of populations: An example from wader populations in the East Asian–Australasian Flyway. Biological Conservation. 143(9):2238-2247
- Clemens RS, Rogers DI, Hansen BD, Gosbell K, Minton CDT, Straw P, Bamford M, Woehler EJ, Milton DA, Weston MA, Venables B, Weller D, Hassell C, Rutherford B, Onton K, Herrod A, Studds CE, Choi CY, Dhanjal-Adams KL, Murray NJ, Skilleter GA, Fuller RA. 2016. Continental-scale decreases in shorebird populations in Australia. Emu. 116:119-135
- Studds CE, Kendall BE, Murray NJ, Wilson HB, Rogers DI, Clemens RS, Gosbell K, Hassell CJ, Jessop R, Melville DS, Milton DA, Minton CDT, Possingham HP, Riegen AC, Straw P, Woehler EJ, Fuller RA. 2017. Rapid population decline in migratory shorebirds relying on Yellow Sea tidal mudflats as stopover sites. Nature Communications. 8:14895
- Wilson HB, Kendall BE, Fuller RA, Milton DA, Possingham HP. 2011. Analyzing variability and the rate of decline of migratory shorebirds in Moreton Bay, Australia. Conservation Biology. 25:758-766
- Murray NJ, Clemens RS, Phinn SR, Possingham HP, Fuller RA. 2014. Tracking the rapid loss of tidal wetlands in the Yellow Sea. Frontiers in Ecology and the Environment. 12:267-272
- Piersma T, Lok T, Chen Y, Hassell CJ, Yang H-Y, Boyle A, Slaymaker M, Chan Y-C, Melville DS, Zhang Z-W, Ma Z. 2016. Simultaneous declines in summer survival of three shorebird species signals a flyway at risk. Journal of Applied Ecology. 53:479-490
- Runge CA, Martin TG, Possingham HP, Willis SG, Fuller RA. 2014. Conserving mobile species. Frontiers in Ecology and the Environment. 12:395–402
- Iwamura T, Possingham HP, Chadès I, Minton C, Murray NJ, Rogers DI, Treml EA, Fuller RA. 2013. Migratory connectivity magnifies the consequences of habitat loss from sea-level rise for shorebird populations. Proceedings of the Royal Society B. 281:20130325
- Finn PG, Catterall CP, Driscoll PV. 2007. Determinants of preferred intertidal feeding habitat for Eastern Curlew: a study at two spatial scales. Austral Ecology. 32:131-144
- Finn PG, Catterall CP, Driscoll PV. 2008. Prey versus substrate as determinants of habitat choice in a feeding shorebird. Estuarine, Coastal and Shelf Science. 80:381-390
- Finn PG. 2007. Feeding ecology and habitat selection. In: Geering A, Agnew L, Harding S (Eds). Shorebirds of Australia. CSIRO Publishing, Victoria. p. 51-59
- Zharikov Y, Milton DA. 2009. Valuing coastal habitats: Predicting high tide roosts of non-breeding migratory shorebirds from landscape composition. Emu. 109:107–120
- Finn PG, Driscoll PV, Catterall CP. 2002. Eastern Curlew numbers at high-tide roosts versus low-tide feeding grounds: A comparison at three spatial scales. Emu. 102:233-239
- Dhanjal-Adams KL, Mustin K, Possingham HP, Fuller RA. 2016. Optimizing disturbance management for wildlife protection: The enforcement allocation problem. Journal of Applied Ecology. 53:1215-1224
- Milton D, Driscoll P. 2006. An assessment of shorebird monitoring in Queensland by the Queensland Wader Study Group. Stilt. 50:242–248
- Blackman JG, Craven SA. 1999. Moreton Bay. In: Blackman JG, Perry TW, Ford GI, Craven SA (Eds). Characteristics of important wetlands in Queensland. Environmental Protection Agency, Queensland. 329-332
- Young PC. 1978. Moreton Bay, Queensland: A nursery area for juvenile penaeid prawns. Australian Journal of Marine and Freshwater Research. 29:55-75
- Piersma T, van Aelst R, Kurk K, Berkhoudt H, Maas LRM. 1998. A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics. Proceedings of the Royal Society B. 265:1377-1383
- Gerritsen AFC, van Heezik YM. 1985. Substrate preference and substrate related foraging behaviour in three Calidris Netherlands Journal of Zoology. 35:671-692
- Rompre G, McNeil R. 1996. Variability in day and night feeding habitat use in the Willet Catoptrophorus semipalmatus during the non-breeding season in northeastern Venezuela. Wader Study Group Bulletin. 81:82-87
- Klein E, Berg EE, Dial R. 2005. Wetland drying and succession across the Kenai Peninsula Lowlands, south-central Alaska. Canadian Journal of Forest Resources. 35:1931–1941
- Barter M. 2002. Shorebirds of the Yellow Sea: importance, threats and conservation status. Wetlands International–Oceania, Canberra
- Moores N. 2006. South Korea’s shorebirds: a review of abundance, distribution, threats and conservation status. Stilt. 50:62–72
- Environment Australia. 1997. The wetlands policy of the Commonwealth Government of Australia. Environment Australia, Canberra
- Finn PG. 2009. Habitat selection, foraging ecology and conservation of eastern curlews on their non-breeding grounds. PhD Thesis. Griffith University, Brisbane
- Fuller RA, Wilson HB, Possingham HP. 2009. Monitoring shorebirds using counts by the Queensland Wader Study Group. Report to Qld Department of Environment and Resource Management. Uniquest, Brisbane, Australia
- Lilleyman A, Franklin DC, Szabo JK, Lawes MJ. 2016. Behavioural responses of migratory shorebirds to disturbance at a high-tide roost. Emu. 116:111-118
- Glover HK, Weston MA, Macguire GS, Miller KK, Christie BA. 2011. Towards ecologically meaningful and socially acceptable buffers: Response distances of shorebirds in Victoria, Australia, to human disturbance. Landscape and Urban Planning. 103:326-334
- Banks PB, Bryant JV. 2007. Four-legged friend or foe? Dog walking displaces native birds from natural areas. Biology Letters. 3:611-613
- Blem CR. 1990. Avian energy storage. Current Ornithology. 7:59-113
- Colwell MA. 2010. Shorebird ecology, conservation, and management. University of California Press, Berkeley and Los Angeles, California, USA
- Hedenström A. 2010. Extreme endurance migration: What is the limit to non-stop flight? PLoS Biology. 8:e1000362
- Landys MM, Ramenofsky M, Wingfield JC. 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. General and Comparative Endocrinology. 148:132-49
- Nudds RL, Bryant DM. 2000. The energetic cost of short flights in birds. Journal of Experimental Biology. 203:1561-1572
- Dolman PM, Sutherland WJ. 1997. Spatial patterns of depletion imposed by foraging vertebrates: Theory, review and meta-analysis. Journal of Animal Ecology. 66:481-494
- Rogers DI. 2003. High-tide roost choice by coastal waders. Wader Study Group Bulletin. 100:73-79
- Fitzpatrick S, Bouchez B. 1998. Effects of recreational disturbance on the foraging behaviour of waders on a rocky beach. Bird Study. 45:157-171
- Paton DC, Ziembicki M, Owen P, Heddle C. 2000. Disturbance distances for water birds and the management of human recreation with special reference to the Coorong region of South Australia. Stilt, 37:46
- Urfi AJ, Goss-Custard JD, Durell SEA l V d. 1996. The ability of oystercatchers Haematopus ostralegus to compensate for lost feeding time: field studies on individually marked birds. Journal of Applied Ecology. 33:873-883
- Rohweder DA, Baverstock PR. 1996. Preliminary investigation of nocturnal habitat use by migratory waders (Order Charadriformes) in northern New South Wales. Wildlife Research. 23:169-184
- Blumstein DT, Fernandez-Juricic E, Zollner PA. Garity SC. 2005. Interspecific variation in avian responses to human disturbance. Journal of Applied Ecology. 42:943-953
- Clemens RS, Skilleter GA, Bancala F, Fuller RA. 2012. Impact of the January 2011 Flood on migratory shorebirds and their prey in Moreton Bay. Report to the Healthy Waterways Partnership. University of Queensland, Brisbane
- Choi C-Y, Coleman J, Klaassen M, Moffitt DJ, Rogers D, Skilleter G, Fuller RA. 2017. Final Report: Migratory Shorebird Monitoring – Understanding Ecological Impact (CA12000284). Report produced for the Ecosystem Research and Monitoring Program Advisory Panel as part of GPC’s Ecosystem Research and Monitoring Program
- Murray NJ, Clemens RS, Phinn SR, Possingham HP, Fuller RA. 2015. Threats to the Yellow Sea’s tidal wetlands. Bulletin of the Ecological Society of America. 96:346-348
- Dhanjal-Adams KL, Klaassen M, Nicol S, Possingham HP, Chadès I, Fuller RA. 2017. Setting conservation priorities for migratory networks under uncertainty. Conservation Biology. 31:646-656
- Klaassen M, Lindström Å, Meltofte H, Piersma T. 2001. Arctic waders are not capital breeders. Nature. 413:794
- Wauchope HS, Shaw JD, Varpe Ø, Lappo EG, Boertmann D, Lanctot RB, Fuller RA. 2017. Rapid climate-driven loss of breeding habitat for Arctic migratory birds. Global Change Biology. 23:1085-1094
- van Gils JA, Lisovski S, Lok T, Meissner W, Ożarowska A, de Fouw J, Rakhimberdiev E, Soloviev MY, Piersma T, Klaassen M. 2016. Body shrinkage due to Arctic warming reduces red knot fitness in tropical wintering range. Science. 352:819-821
- Queensland Government. 2015. Queensland Government population projections, 2015 edition. Queensland Government Statistician’s Office, Brisbane
- Dhanjal-Adams KL, Hanson JO, Murray NJ, Phinn SR, Wingate VR, Mustin K, Lee JR, Allan JR, Cappadonna JL, Studds CE, Clemens RS, Roelfsema CM, Fuller RA. 2016. The distribution and protection of intertidal habitats in Australia. Emu. 116:208-214
- Hansen BD, Fuller RA, Watkins D, Rogers DI, Clemens RS, Newman M, Woehler EJ, Weller DR. 2016. Revision of the East Asian-Australasian Flyway Population Estimates for 37 listed Migratory Shorebird Species. Report for the Department of the Environment. BirdLife Australia, Melbourne
